Credit: Unsplash/CC0 Public Domain
A randomized controlled trial evaluates the safety of furosemide in preterm infants at risk of bronchopulmonary dysplasia. Findings from the study will be presented during the Pediatric Academic Societies (PAS) 2022 Meeting, taking place April 21-25 in Denver.
The objective of the study was to evaluate the safety and preliminary efficacy of furosemide in preterm infants at risk of developing bronchopulmonary dysplasia.
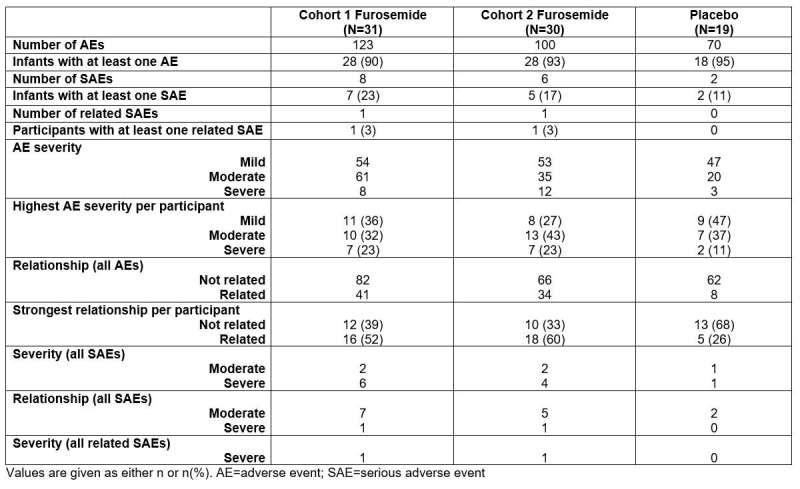
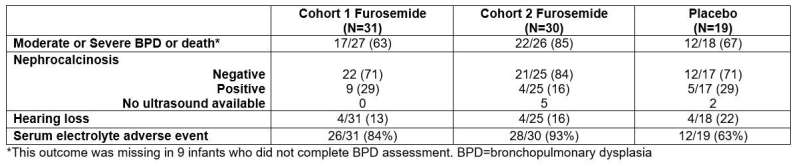
Researchers found that in preterm infants at high risk for bronchopulmonary dysplasia, adverse events occurred in nearly all infants regardless of treatment group. Furosemide increased the risk of electrolyte adverse events. There was no difference in hearing loss, nephrocalcinosis, or bronchopulmonary dysplasia/death.
"The drug furosemide is commonly used in premature infants hospitalized in the neonatal intensive care unit to prevent bronchopulmonary dysplasia, a type of chronic lung disease," said Rachel G. Greenberg, MD, MB, MHS, associate professor of pediatrics with the division of neonatal-perinatal medicine at Duke University Medical Center, and program director with Duke Neonatal-Perinatal Medicine Fellowship. "Unfortunately, very little data is available to help neonatologists understand whether furosemide is safe and effective. Our study, which was performed at 17 centers within the NICHD Pediatric Trials Network, was the first randomized controlled trial to evaluate the safety and preliminary efficacy of furosemide in premature infants at risk for developing bronchopulmonary dysplasia."
-
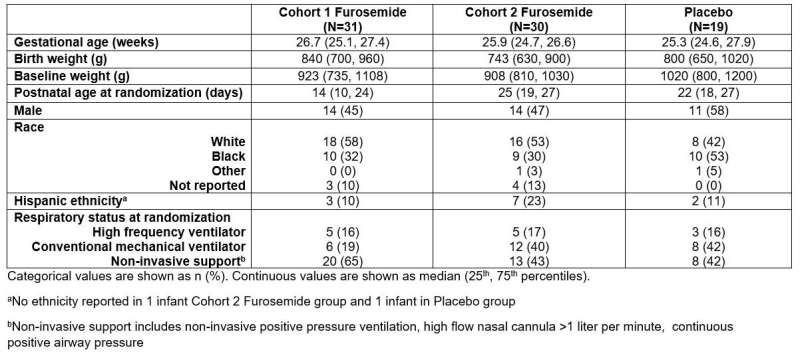
Demographics and clinical characteristics. Credit: Duke Clinical Research Institute
-
Adverse events. Credit: Duke Clinical Research Institute
-
Secondary end points. Credit: Duke Clinical Research Institute
Dr. Greenberg added that they "found that infants exposed to furosemide had more electrolyte problems but no greater risk of overall safety events, including kidney stones and hearing problems. The results of this study will help the practicing neonatal provider and help to design future trials."
More information: Conference: www.pas-meeting.org/
Provided by American Pediatric Society