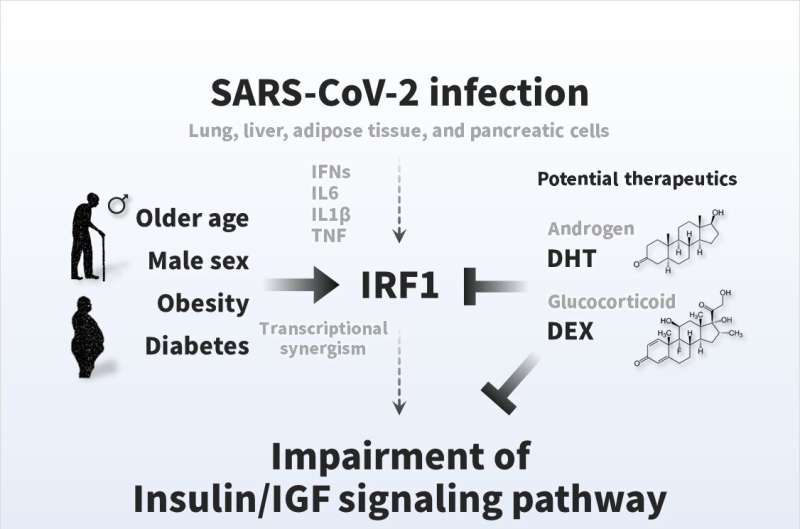
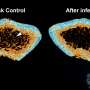
Pathogenesis of COVID-19 symptoms and therapeutic strategies. The insulin/IGF signaling pathway plays an important role in many biological processes, such as energy metabolism and cell survival. SARS-CoV-2 infection impairs transcriptional expression of the insulin/IGF signaling pathway in the host lung, liver adipose tissue, and pancreatic cells, which is likely attributed to interferon regulatory factor 1 (IRF1). The pathological trait is aggravated in whole blood, a systemic indicator, of critical patients with COVID-19 with exacerbated cell damage, cell death, and metabolic abnormalities, which could be ameliorated by androgen (DHT) and/or glucocorticoid (DEX) interventions. Higher basal IRF1 expression by pathological (older age, male sex, obesity, and diabetes) reasons in respiratory, metabolic, and/or endocrine organs might contribute to synergistic upregulation of IRF1 in response to SARS-CoV-2 infection, which may make the people more vulnerable to COVID-19. Credit: Osaka University, CC BY
It has become abundantly clear that coronavirus disease 19 (COVID-19), despite being transmitted by breathing in the SARS-CoV-2 virus, can have harmful effects far beyond the lungs. Now, researchers from Japan have identified a pivotal gene that mediates the effects of SARS-CoV-2 infection on blood sugar metabolism.
In a study published in June in Metabolism, researchers from Osaka University reveal that COVID-19 can cause metabolism problems, and sometimes even diabetes, by interfering with insulin signaling.
COVID-19 is best known for causing respiratory disease, but can also damage other organ systems; notably, disruption of blood sugar regulation can lead to new-onset diabetes. However, it is unclear how infection with the SARS-CoV-2 virus results in these effects.
"The insulin/IGF signaling pathway is a key pathway in the regulation of energy metabolism and cell survival," says Jihoon Shin, first author on the study. "Therefore, we suspected that SARS-CoV-2 affects this signaling pathway to cause problems with blood sugar regulation."
To test this, the researchers analyzed datasets of gene expression from patients, as well as in vivo and in vitro models, infected with SARS-CoV-2. They specifically looked for genes that were noticeably over- or under-expressed compared with uninfected patients, animals, or cells.
"The results were striking," states Iichiro Shimomura, senior author of the study. "Infection with SARS-CoV-2 affected the expression of insulin/IGF signaling pathway components in the lung, liver, adipose tissue, and pancreatic cells. Moreover, these changes were attributed in part to activation of interferon regulatory factor 1 (IRF1)."
Further investigation showed that IRF1 expression is elevated in older patients, men, obese individuals, and patients with diabetes. The synergistic effect of older age, male sex, obesity and diabetes with SARS-CoV-2 means that the expression of IRF1 occurs at an increased rate, which may explain why these patients are more vulnerable to COVID-19. In addition, critical patients with COVID-19 had higher IRF1 expression and lower insulin/IGF signaling pathway genes in their blood compared with noncritical patients. Finally, treating SARS-CoV-2–infected cells or an animal model with hormonal factors that decreased IRF1 expression enhanced insulin/IGF signaling.
"Our findings suggest that SARS-CoV-2 infection impairs insulin/IGF signaling by increasing IRF1 expression, thereby disrupting blood sugar metabolism. Decreasing IRF1 expression by treatment with factors such as dihydrotestosterone and dexamethasone could help mitigate the effects of COVID-19," says Shin.
Given the devastating impact that COVID-19 can have on multiple organ systems, treatment strategies that could decrease the effect of the disease on blood sugar metabolism could be vitally important. By identifying patients at greater risk of experiencing these effects and intervening to decrease IRF1 activation, some of the severe consequences of COVID-19 could be avoided in susceptible populations.
More information: Jihoon Shin et al, SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1, Metabolism (2022). DOI: 10.1016/j.metabol.2022.155236
Provided by Osaka University