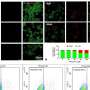
mRNA-N vaccination induced protection against SARS-CoV-2 challenge in mice and hamsters. (A) Mouse experimental design and timeline. Two groups of BALB/c mice (n = 8) were intramuscularly vaccinated with PBS (mock) or mRNA-N vaccine (1 μg) at weeks 0 and 3. Two weeks after booster vaccination (week 5), mice were intranasally challenged with mouse-adapted (MA) SARS-CoV-2 (2 × 104 pfu). Two days post infection (DPI), viral loads in the lungs were analyzed to evaluate vaccine-induced protection. (B) Comparison of viral RNA copies in the mouse lungs between mock and vaccine groups are shown. Viral RNA copies were quantified by RT-PCR and expressed as log10 copies per milligram of lung tissue. (C) Comparison of viral titers in the mouse lungs between mock and vaccine group are shown. Viral titers were quantified by plaque assay and expressed as log10 FFU per gram of lung tissue. (D) Hamster experimental design and timeline. Three groups of hamsters were investigated. The first two groups (n = 12 per group) were intramuscularly vaccinated with mock or mRNA-N (2 μg) at weeks 0 and 3, followed by SARS-CoV-2 Delta challenge at week 5 and viral load analysis on 2 (n = 6) and 4 (n = 6) DPI. The third group (n = 6) received the same mRNA-N vaccine and subsequent viral challenge, except that these hamsters were intraperitoneally injected with two doses of antibodies for CD8+ T cell depletion at 6 and 3 days before viral challenge. Viral loads were analyzed on 2 DPI (n = 6). (E) Comparison of viral RNA copies in hamster lungs (log10 viral copies per milligram) between mock and vaccine group are shown for samples collected on 2 and 4 DPI. (F) Comparisons of viral titers in the hamster lungs (log10 FFU per gram) between mock and vaccine group are shown for samples collected on 2 and 4 DPI. (G) Comparison of hamster body weight loss is shown for the mock and vaccine group from days 0 to 4 DPI. (H) A comparison of viral RNA copies in the lung of hamsters (log10 viral copies per milligram) among the three groups is shown for samples collected on 2 DPI. The dashed line in (F) indicates the limit of detection. Data are presented as median and IQR where appropriate. Mann-Whitney (B, C, and G) or Kruskal-Wallis (E, F, and H) test was used for statistical analysis. *P < 0.05, **P < 0.01, and ***P < 0.001. Credit: Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abq1945
A large team of researchers at the University of Texas Medical Branch, working with two colleagues from the University of Pennsylvania Perelman School of Medicine and two with the Mayo Clinic, has developed a vaccine for COVID-19 that targets both the spike and nucleocapsid proteins in the SARS-CoV-2 virus. In their paper published in the journal Science Translational Medicine, the group explains their approach to developing the new vaccine and describes how well it worked in test mice and hamsters.
As the pandemic has worn on, medical scientists have come to learn a lot about the virus behind COVID-19 infections. To that end, they have developed vaccines that target spike proteins on the virus that play a major role in allowing entry to host cells. Unfortunately, such spike proteins are easily changed by the virus, which results in the development of variants and the need for updated vaccines. In this new effort, the researchers focused on another protein in the virus, one that is less adaptable.
Just as the spike protein in the virus has been shortened to "S," the protein drawing the focus of the researchers on this new effort is shortened to "N"—for nucleocapsid protein. It is located in the core of the virus and helps it to replicate. Due to its function and location, it is much less likely to change, which means variants with different N proteins are not likely to develop very quickly.
The researchers created an mRNA-type vaccine that targets the N protein and tried it out on test mice. They found, as expected, that it did not do much to prevent new infections. But it did incite a strong T-cell response, which is good, because such cells help to clear the virus from the body.
The researchers then created an mRNA-type vaccine that focused on both the S and N proteins and then injected it into test mice. They observed both a very strong immune response and a quick recovery period. They also found the virus was less able to damage the lungs. And the bivalent vaccine worked better than a strictly S-based vaccine in a control group. The team ran similar tests with hamsters and found similar results.
More information: Renee L. Hajnik et al, Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abq1945
Journal information: Science Translational Medicine
© 2022 Science X Network