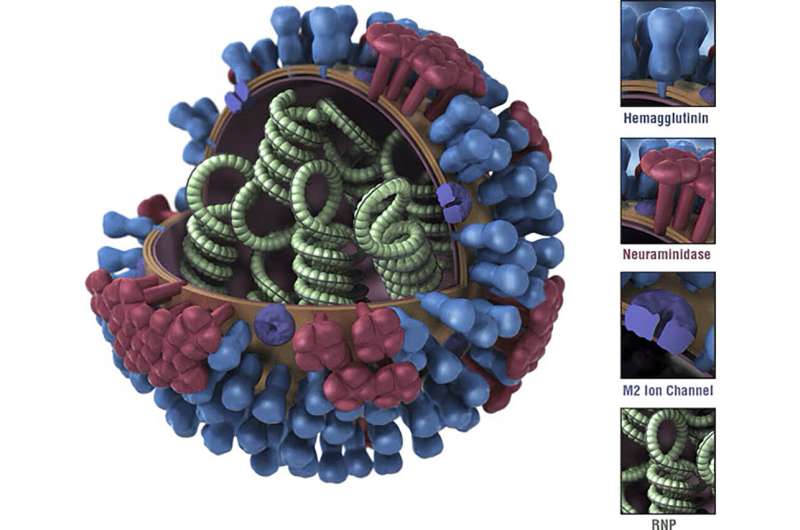
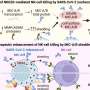
A schematic diagram of the influenza virus, showing the surface proteins hemagglutinin (blue, peanut shaped) and neuraminidase (red, flower shaped), to which antibodies attach during an immune response. A new vaccine from Duke helps the immune system target the stalk of the hemagglutinin protein, rather than its top. Credit: U.S. Centers for Disease Control
Duke researchers have opened a new avenue in the attack against influenza viruses by creating a vaccine that encourages the immune system to target a portion of the virus surface that is less variable.
Their approach worked well in experiments with mice and ferrets and may lead to more broadly protective influenza vaccines and less reliance on an annual shot tailored to that year's versions of the virus. Even with vaccines, influenza kills about a half-million people each year around the world.
This new vaccine approach, described May 1 in the journal Science Translational Medicine, is part of a five-year-old effort to develop a longer-lasting universal flu vaccine that would be able to foil all versions of the virus.
Influenza strains are referred to by a shorthand code, H5N1 for example, that describes which flavors of two particular surface proteins it carries. The H (sometimes HA), is hemagglutinin, a lollipop-shaped protein that binds to a receptor on a human cell, the first step toward getting the virus inside the cell. The N is neuraminidase, a second protein that enables a newly made virus to escape the host cell and go on to infect other cells.
"On the virus particle, there's five to 10 times more hemagglutinin than neuraminidase," said Nicholas Heaton, Ph.D., an associate professor of molecular genetics and microbiology at Duke who led the research.
"If we took your blood to see if are you likely to be protected from a strain of flu, we'd be measuring what your antibodies do to hemagglutinin as the best metric of what's likely to happen to you. The strongest correlates of protection have to do with hemagglutinin-directed immunity."
Credit: Duke University
Vaccines teach the immune system to react to pieces of the virus that have been specifically tailored to the versions of influenza that are expected to be the most threatening in the coming flu season. The reason we need a new flu shot every fall isn't because the vaccine wears out; it's because the influenza virus is constantly changing the surface proteins that vaccines target.
Flu shots—and immune systems—tend to target the bulb-like "head" of hemagglutinin rather than the stalk. But the details of that head region also change constantly, creating an arms race between vaccine design and viruses. The stalk, by comparison, changes much less.
"A number of groups have gone through and experimentally mutagenized the whole hemagglutinin and asked 'which areas can change and still allow the hemagglutinin to function?'" Heaton explained. "And the answer is, you can't really change the stalk and expect it to continue to function."
So the Duke team sought to design proteins that elicit an immune response more focused on the stalk rather than the head. "The virus has evolved to have the immune system recognize these (features on the head region). But these are the shapes the virus can change. That is an insidious strategy," Heaton said.
Using gene-editing, they created more than 80,000 variations of the hemagglutinin protein with changes in one portion right on the top of the head domain and then tested a vaccine filled with a mixture of these variations on mice and ferrets.
Because of the broad variety of head conformations being presented to the immune system and the relative consistency of the stalks, these vaccines produced more antibodies to the stalk portion of hemagglutinin in response. "The opportunity for the immune system to see that (head portion) over and over and over like it needs to is compromised because there's diversity there," Heaton said.
In lab tests and animals, the experimental vaccine caused the immune system to respond more strongly to stalk regions because they stayed consistent. This boosted the immune response to the vaccine overall, and in some cases, even improved antibody responses to the head region of the protein as well.
"Antibodies against the stalk work differently," Heaton said. "Their mechanism of protection is not necessarily to block the first step of infection. So then our idea was, "What if we can come up with a vaccine that gives us both? What if we can get good head antibodies and at the same time also get stalk antibodies in case the vaccine selection was wrong, or if there's a pandemic?'"
"Essentially, the paper says, Yes, we can accomplish that," Heaton said.
After a shot of the highly variant vaccine was administered in some experiments, 100% of the mice avoided illness or death from what should have been a lethal dose of flu viruses.
The next steps of the research will attempt to understand whether the same level of immunity can be achieved by presenting fewer than 80,000 hemagglutinin variants.
More information: Zhaochen Luo et al, Vaccination with antigenically complex hemagglutinin mixtures confers broad protection from influenza disease, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.adj4685. www.science.org/doi/10.1126/scitranslmed.adj4685
Journal information: Science Translational Medicine
Provided by Duke University