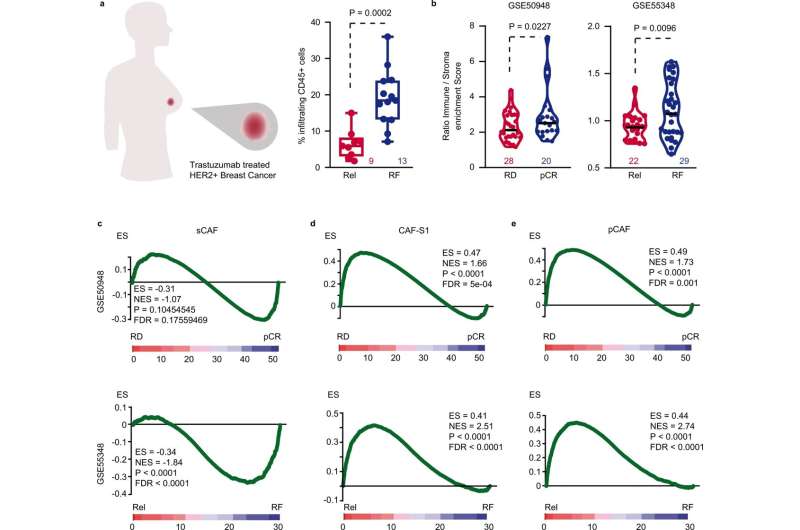
Stromal traits define resistance to trastuzumab. a Percentage of CD45+ cells in HER2+ BC tumors comparing relapse-free (blue) to relapsing patients (red) after trastuzumab-based therapy. Central mark indicates the median, box extends from the 25 to 75th percentiles, whiskers represent the maximum and minimum data point. Number of patients in each group is indicated. b Violin plots of immune/stroma enrichment score ratio for patients with pCR (blue) vs. RD (red) (GSE50948) and for relapsing (red) vs. relapse-free patients (blue) (GSE55348). Number of patients in each group is indicated. c–e Gene-set enrichment analyses (GSEA) in tumor samples collected before treatment of (c) sCAF, (d) CAF-S1, and (e) pCAF gene expression signatures comparing pCR vs. RD (upper row) and relapsing vs. relapse-free (lower row) HER2+ BC after trastuzumab treatment. Rel relapsing patients, RF relapse-free patients, pCR pathological complete response, RD residual disease, ES enrichment score, NES normalized enrichment score, FDR false discovery rate. Two-sided, unpaired t test p values (P) are indicated for (a, b). GSEA nominal p value (P) and FDR-adjusted p value are indicated for (c–e). Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-32782-3
The microenvironment surrounding tumors in HER2+ breast cancer protects them and helps them develop resistance to the most widely used treatment, the monoclonal antibody trastuzumab. And a particular type of cell in this microenvironment, fibroblasts, plays a key role in this process. These cells have the ability to block the immune system and thereby protect the tumor. Finding a way to overcome this boosts the treatment's capacity to kill tumor cells.
Specifically, it is the presence of TGF-beta-activated fibroblasts, which express a molecule called FAP, that protects the tumor from the action of immune cells. Trastuzumab has the ability to target cancer cells that present high levels of the HER2 protein, and when it binds to the cancer, it activates a strong immune response, which is a major contributor to its efficacy against the tumor.
However, in many tumors, the immune system is unable to break through the microenvironment surrounding the tumor to eliminate it. This leads to treatment resistance and increases the capacity of this type of cancer to evade the drug and proliferate further. This mechanism was discovered by a team of researchers from the IMIM-Hospital del Mar and the CIBER Cancer Research Center (CIBERONC) in a study that has been published in the journal Nature Communications.
The authors have also identified a way to overcome the tumor's ability to protect itself and allow the immune system to act on the tumor cells. Using an ex vivo model, i.e., a model that includes living cells from breast cancer patients, the researchers have shown that by targeting the fibroblast-expressed FAP molecules with immunotherapy, this ability to prevent access by immune cells can be reversed.
"When this molecule, FAP-IL2v, is added to a tumor recreated ex vivo that contains this treatment-resistant microenvironment, in contact with immune cells, trastuzumab's effectiveness is restored," states Dr. Alexandre Calon, senior author of the research and head of the Translational Research in Tumor Microenvironment Laboratory at the IMIM-Hospital del Mar. It should be noted that the model generated uses human cells and is also applicable to other types of tumors.
The study has validated the results with three cohorts of patients and more than 120 samples. In all of them, the levels of fibroblast activation were found to be directly related to the immune system's ability to act on the tumor. The higher the levels, the greater the difficulty in accessing and eliminating tumor cells despite the action of trastuzumab. Dr. Calon emphasized that this facilitates a better selection of patients who will benefit from FAP-IL2v treatment aimed at deactivating the action of the tumor microenvironment.
"If we filter people based on these characteristics, we can isolate a population of treatment-resistant patients who can be targeted with this molecule to restore the effectiveness of the breast cancer therapy," he explains.
There are already drugs available that can be used to achieve this effect, although further studies must be carried out to evaluate their application in patients, as Dr. Joan Albanell, head of the Oncology Department at the Hospital del Mar, Director of the Cancer Research Program at the IMIM-Hospital del Mar and co-author of the study, points out.
"The study identifies tumors in which anti-HER2 therapy resistance is caused primarily by one type of fibroblast rather than other causes. This important discovery should be used to design clinical trials with drugs that overcome this resistance only for those patients in whom this resistance is operative. This is where we need to move towards precision oncology," adds Dr. Albanell.
More information: Elisa I. Rivas et al, Targeted immunotherapy against distinct cancer-associated fibroblasts overcomes treatment resistance in refractory HER2+ breast tumors, Nature Communications (2022). DOI: 10.1038/s41467-022-32782-3
Journal information: Nature Communications
Provided by IMIM (Hospital del Mar Medical Research Institute)