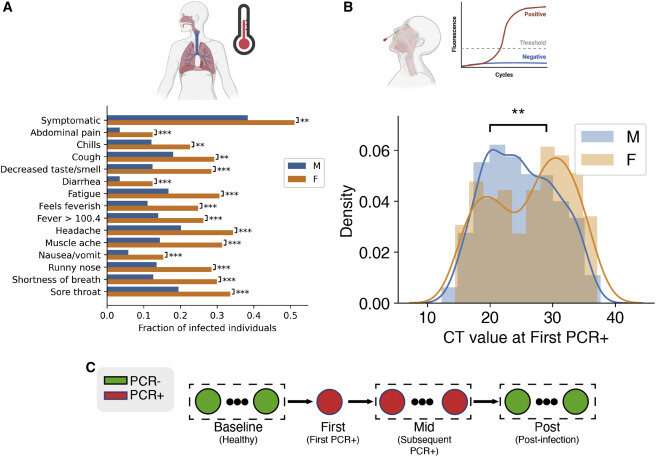
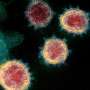
Analysis of sex divergence in clinical measurements. (A) The fraction of each reported symptom for infected females (n = 137) and males (n = 1,033). Statistical significance was tested using the chi2 independence test. Statistical significance: ∗∗∗p = less than 0.001, ∗∗p = less than 0.01, ∗p = less than 0.05. (B) Initial viral load, measured by probe CT and averaged across the S, N, and ORF1ab genes, between females and males. Statistical significance was tested by the Kolmogorov-Smirnov test. (C) Clinical data and biospecimens were collected longitudinally for each subject. Samples were labeled “baseline” if they tested negative for SARS-CoV-2, the first PCR-positive sample from each infected individual was labeled “first,” and any subsequent PCR-positive sample was labeled “mid.” Subjects were followed throughout infection, and any PCR-negative samples taken after infection were labeled “post.” Credit: Cell Systems (2022). DOI: 10.1016/j.cels.2022.10.005
Significant immunological differences between young men and women were shown to mediate sex differences in the responses to the virus that causes COVID-19, according to a collaborative study of nearly 3,000 young, healthy members of the U.S. Marine Corps. The study was conducted by researchers at the Icahn School of Medicine at Mount Sinai, Princeton University, and the Naval Medical Research Center, published November 7 in Cell Systems.
"Through a well-controlled longitudinal study of young Marine recruits, we were able to identify sex differences across many metrics, including symptoms, viral load, blood transcriptome, RNA splicing, and proteomic signatures," said Stuart Sealfon, MD, the Sara B. and Seth M. Glickenhaus Professor of Neurology at Icahn Mount Sinai and co-senior author of the study. "We found that females have higher pre-infection antiviral interferon-stimulated gene (ISG) expression, a wide array of genes that generally function to inhibit viral replication. Our results indicate that these ISG differences could mediate sex differences in response to virus infection."
COVID-19, which has led to more than 6.5 million deaths worldwide, on average yields worse outcomes in males. Innate and adaptive immune responses to infection with SARS-CoV-2, the virus causing COVID-19, are different in males and females. These differences in response and outcomes could be influenced by pre-existing factors that either worsen the disease in males (such as androgen effects or the prevalence of comorbidities), or that improve the outcome in females (such as immune system effectiveness).
"The identification of unique signatures between the sexes will help inform the design of future medical countermeasures that can prevent and treat SARS-CoV-2 infections not only in military recruits but improve global public health as well," said Cmdr. Andrew Letizia, MD, Deputy Director of the Naval Medical Research Center's infection disease directorate and an author of the study.
To understand the basis of these sex differences, the study team analyzed data collected from a cohort of new Marine recruits in a controlled setting as they began military training. Through the prospective COVID-19 Health Action Response for Marines (CHARM) study, a total of 2,641 males and 244 females who were initially SARS-CoV-2-seronegative were followed longitudinally with symptom screening, serial swab PCR testing for SARS-CoV-2, and blood sampling for molecular analyses. During the 12 weeks after study entry, which included two weeks of supervised quarantine and 10 weeks of recruit training, a total of 1,033 males and 137 females tested positive for SARS-CoV-2.
The study was conducted between May and September of 2020, prior to the release of vaccines and treatments specifically directed at SARS-CoV-2, and none of the participants were enrolled in other clinical trials at the time. This collaboration of academic and military scientists and the Marine Corps permitted identification and a causal analysis of pre-existing immune system differences and their significance in molecular and clinical sex differences observed during SARS-CoV-2 infection.
Using RNA sequencing and clinical measure analysis, the research team found that infected females had higher rates of symptoms, yet their average viral load was 2.6 times lower than that of infected males. They also identified sex-specific molecular signatures for gene expression, alternative splicing, and immunoproteomics (the study of large sets of proteins involved in the immune response).
Specifically, differential splicing of 594 sites was found during infection only in males, whereas 376 genes and 270 sites were modulated only in females. Many of these immune response genes are sex-biased or sex-specific, and there are more genes in the enriched immune response set in females than males across both. This suggests a broadly stronger transcriptional and post-transcriptional immune response to acute SARS-CoV-2 infection in females.
"Sex-specific responses to COVID19 are notoriously challenging to study due to the many confounding variables, including co-morbidities, differences in environment, fitness etc. CHARM's unique, well-controlled, longitudinally sampled cohort combined with computational analysis is what enabled identification of associated, sex-specific molecular signatures that are present prior to infection," said Olga Troyanskaya, Professor of Computer Science at Princeton University, Associate Director for Genomics at the Flatiron Institute of the Simons Foundation, and co-senior author of the study.
The authors note some limitations to their study, including that the cohort was mainly young, healthy adults and did not include any cases of severe COVID-19, which allowed them to tightly control for baseline health levels but limited their ability to reach definitive conclusions about the relevance of these findings to older or less healthy individuals or to the development of more severe COVID-19.
The large, relatively homogenous cohort of young Marine recruits exposed to the virus under similar recruit training conditions minimized the influence of confounding factors like age, comorbidities, race, ethnicity, and environmental exposures, which allowed identification of the causal contribution of baseline immunological sex differences to the molecular responses and symptoms caused by SARS-CoV-2 infection.
More information: Natalie Sauerwald et al, Pre-infection antiviral innate immunity contributes to sex differences in SARS-CoV-2 infection, Cell Systems (2022). DOI: 10.1016/j.cels.2022.10.005
Journal information: Cell Systems
Provided by The Mount Sinai Hospital