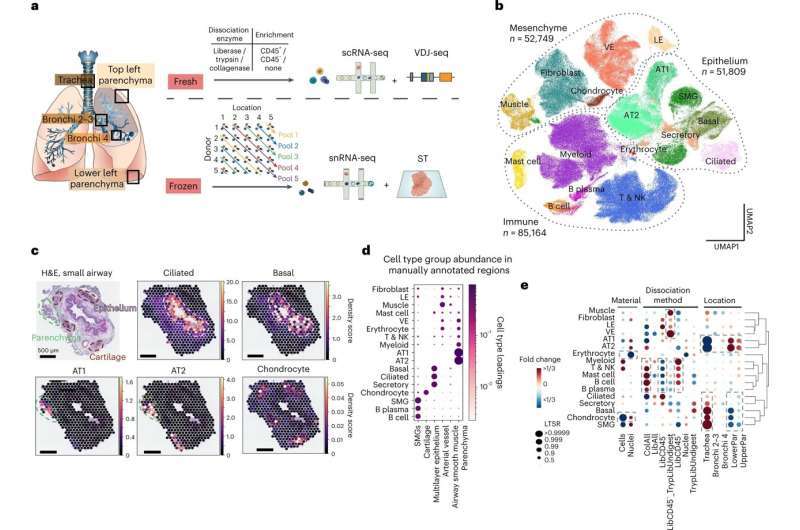
Spatial multi-omics atlas of the human lung allows the identification of cell types and their location. a, Multi-omics spatial lung atlas experimental design included fresh and frozen sampling from five locations for scRNA-seq (seven donors), sc VDJ-seq (four donors), snRNA-seq (seven donors) and Visium ST (seven donors). Five donors (D) from the frozen samples were pooled into five reactions, each containing different locations (Loc) from donors. b, UMAP of all scRNA-seq/snRNA-seq from 193,108 cells/nuclei in total from ten donors. Cells from all major subsets were captured. c, cell2location mapping on Visium ST from a bronchi section shows matching of cell types to expected structures. H&E staining and cell abundance estimated by cell2location (density score) for ciliated, basal epithelium, AT1, AT2 and chondrocyte cell types with histology image in the background. Dotted lines circle the epithelium (pink), parenchyma (green) and cartilage (brown). d, Cell type groups are enriched in expected micro-anatomical tissue environments on Visium ST across sections from five donors. Cell type loadings are represented by both dot size and color for cell types annotated in b across the manually annotated micro-environments in the Visium data. e, Cell type capture is affected by protocol and location. Cell type proportion analysis with fold changes and LTSR score for all cell type groups with regard to the material, protocol and location. Dashed boxes highlight the greatest changes. AT1, alveolar type 1; AT2, alveolar type 2; LE, lymphatic endothelium; VE, vascular endothelium. Credit: Nature Genetics (2022). DOI: 10.1038/s41588-022-01243-4
The most comprehensive lung cell atlas to date, from the Wellcome Sanger Institute and collaborators, has revealed 11 new lung cell types and offers detailed insight into an immune process involved in fighting lung infections.
Published today in Nature Genetics, this freely available resource highlights multiple immune cells, barrier cells, and their environments in the lung that are implicated in respiratory diseases and infections.
This new lung cell atlas, which is part of the wider international Human Cell Atlas Initiative, combined single cell sequencing with spatial transcriptomics to provide a fuller picture of how cells interact and communicate with each other.
While single cell studies have advanced the understanding of lung function, the lungs are made up of complex structures and environments that cannot be investigated by single cell sequencing alone. For example, there are many unanswered questions about how the cells are organized and how specific cell types, especially rare cell types, contribute to lung disease.
Chronic lung diseases, such as chronic obstructive pulmonary disease (COPD) and interstitial lung disease, are leading causes of death worldwide. Understanding communication between cells within their local environment in healthy lungs can help determine what is disrupted in disease, and give clues on how to prevent or treat this.
In this study, researchers from the Wellcome Sanger Institute and collaborators, genetically profiled nearly 200,000 cells from lung tissue of 13 donors, discovering 11 new cell types, and showing the exact location of 80 cell types in total.
Of these new cell types, peribronchial fibroblasts were found to be implicated in COPD and idiopathic pulmonary fibrosis. While further research is required to investigate how these cells are involved, this discovery demonstrates the potential of using this lung atlas to uncover new links between cell pathways and disease.
Dr. Elo Madissoon, joint first author and Post-Doctoral Fellow at the Wellcome Sanger Institute and EMBL's European Bioinformatics Institute (EMBL-EBI), said, "By being able to analyze multiple locations of the same lung, we were able to get key information about a range of cells in a single study, many of which were not previously mapped. In addition, the link we found between peribronchial fibroblasts and chronic lung conditions shows how this atlas goes beyond reference data and can offer new insights into disease."
In addition to this, the researchers were able to define a lung microenvironment which they call the gland associated immune niche (GAIN). The GAIN exists to help fight respiratory infections and ultimately promotes IgA antibody production. This is the first time research has managed to map this complex process in detail.
IgA responses are important for efficient protection against respiratory infections and are impaired in lung diseases, such as COPD and cystic fibrosis. Further research investigating GAIN function could help develop therapies to alleviate disease symptoms, improve resistance to infection or create ways to boost vaccine responses.
Dr. Amanda Oliver, joint first author and Postdoctoral Research Fellow at the Wellcome Sanger Institute, said, "Our study provides unique information about how cells communicate in the human lung and airways. By integrating single cell and spatial data, we were able to study how epithelial, endothelial and immune cells interact to form an immune niche—the GAIN—which is likely to be important for protection against respiratory infections. Our single cell data allows us to drill into the signaling circuits that enable this immune niche to function and could be crucial in developing new ways to treat disease in the future."
Dr. Kerstin Meyer, joint senior author and Principal Staff Scientist at the Wellcome Sanger Institute, said, "Our freely available comprehensive lung atlas not only genetically profiles 80 cell types, including 11 new ones, it starts to describe how these communicate with each other. Our research builds on the single cell era and by including spatial data, we have begun to see how lung cells interact in their specific microenvironments. Understanding how lung cells interact with each other in a healthy lung is crucial if we hope to identify where something has gone wrong to cause disease. Our atlas is a valuable resource for the scientific community and we look forward to larger studies that will continue to piece together the full puzzle of how the lung works at a cellular level."
Dr. Sarah Teichmann, joint senior author, Head of Cellular Genetics at the Wellcome Sanger Institute and Co-Founder of the Human Cell Atlas, said, "A detailed understanding of cells through the Human Cell Atlas will help explain many aspects of human health and disease. Our lung cell atlas gives a more in depth insight into the functioning of a vital organ, and is an important contribution towards the complete Human Cell Atlas. In addition to this, our work could help identify which pathways or cells in the lung could be targeted to help improve immunity in individuals with impaired lung function. We would like to thank the organ donors, as well as their families and loved ones for making this research possible."
More information: Madissoon, E. et al, A spatially resolved atlas of the human lung characterizes a gland-associated immune niche, Nature Genetics (2022). DOI: 10.1038/s41588-022-01243-4. www.nature.com/articles/s41588-022-01243-4
Journal information: Nature Genetics
Provided by Wellcome Trust Sanger Institute