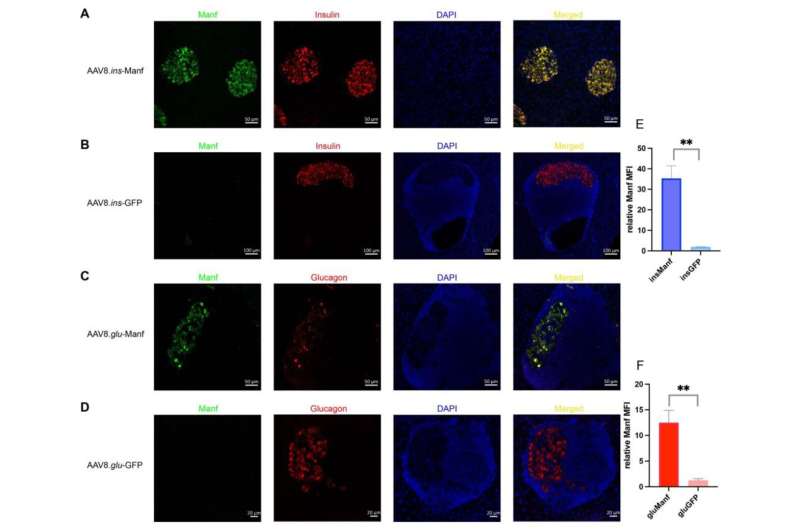
Islets of AAV8.ins-Manf- or AAV8.glu-Manf-treated mice express Manf. (A–D) AAV8.ins-Manf-, AAV8.ins-GFP-, AAV8.glu-Manf-, and AAV8.glu-GFP-treated mice were stained with antibodies: Manf (green), insulin (red), or glucagon (red) and DAPI (blue). (E,F) mean fluorescence intensity (MFI) of Manf in islets of AAV8.ins-Manf-, AAV8.ins-GFP-, AAV8.glu-Manf-, and AAV8.glu-GFP-treated mice. Three to four slides from each mouse (in total 4 mice per group) were stained with antibodies: Manf, insulin, or glucagon and DAPI. Confocal images were captured and analyzed using ImageJ software for determining MFI. Results are presented in means ± SEM (n = 4 per group). Unpaired t-tests were performed for comparison, ** denote p < 0.05. Credit: Biomolecules (2022). DOI: 10.3390/biom12101493
Researchers from the Liston lab, at the Babraham Institute, have recently published details about a preventative therapeutic for diabetes in mice. The team has been able to prevent diabetes in mice by manipulating signaling pathways in pancreatic cells to prevent stress-induced cell death. The treatment targets a pathway common to both major types of diabetes and therefore could have huge therapeutic potential once translated into a clinical treatment.
Dr. Kailsah Singh, former research fellow in the Liston lab, described their findings: "Our results show that MANF could prevent the beta cell damage by preventing the inflammation in islets, which is a hallmark of type 1 diabetes."
For over 35 years there have been failed attempts to prevent type 1 diabetes development. Previous approaches have sought to target the autoimmune nature of the disease, but Dr. Adrian Liston, senior Group Leader in the Immunology research program, wanted to investigate if there was more causing the deterioration in later stages than just the immune response.
The Liston lab sought to understand the role of cell death in the development of diabetes and therefore approached this problem by identifying the pathways that decide whether stressed insulin-producing cells of the pancreas live or die, and therefore determine the development of disease.
Their hope was to find a way to stop this stress-related death, preventing the decline into diabetes without the need to focus solely on the immune system. First, the researchers had to know which pathways would influence the decision of life or death for the beta cell.
In previous research, they were able to identify Manf as a protective protein against stress induced cell death, and Glis3 which sets the level of Manf in the cells. While type 1 and 2 diabetes in patients usually have different causes and different genetics, the GLIS3-MANF pathway is a common feature for both conditions and therefore an attractive target for treatments.
In order to manipulate the Manf pathway, the researchers developed a gene delivery system based on a modified virus known as an AAV gene delivery system. The AAV targets beta cells, and allows these cells to make more of the pro-survival protein Manf, tipping the life-or-death decision in favor of continued survival.
To test their treatment, the researchers treated mice susceptible to spontaneous development of autoimmune diabetes. Treating pre-diabetic mice resulted in a lower rate of diabetes development from 58% to 18%. This research in mice is a key first step in the development of treatments for human patients.
"A key advantage of targeting this particular pathway is the high likelihood that it works in both type 1 and type 2 diabetes," explains Dr. Adrian Liston.
"In type 2 diabetes, while the initial problem is insulin-insensitivity in the liver, most of the severe complications arise in patients where the beta cells of the pancreas have been chronically stressed by the need to make more and more insulin. By treating early type 2 diabetes with this approach, or a similar one, we have the potential to block progression to the major adverse events in late-stage type 2 diabetes."
The paper is published in the journal Biomolecules.
More information: Kailash Singh et al, Gene Delivery of Manf to Beta-Cells of the Pancreatic Islets Protects NOD Mice from Type 1 Diabetes Development, Biomolecules (2022). DOI: 10.3390/biom12101493
Provided by Babraham Institute