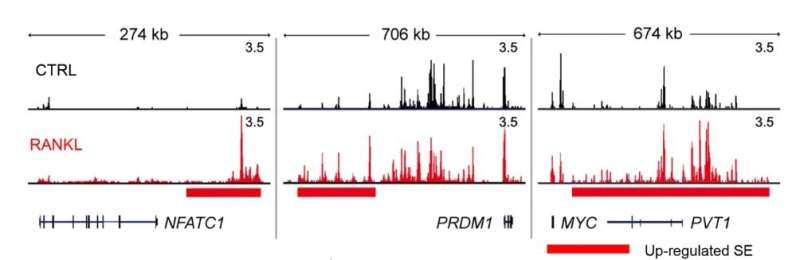
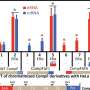
Shown above are the representative tracks of H3K27ac ChIP-seq at NFATc1, PRDM1, and MYC loci under the indicated conditions. Credit: UNIST
A research team, led by Professor Sung Ho Park in the Department of Biological Sciences at UNIST announced the results of a study on osteoblasts that damage joint bones in patients with rheumatoid arthritis.
In this study, the research team studied the possibility of a treatment method targeting mechanisms related to the differentiation process of osteoblasts that melt bones through enzyme reactions. First, it was confirmed that a super-enhancer is formed near the NFATC1 gene, which is known to be an important factor in the formation of osteoblasts, and it is formed only in osteoblasts.
In addition, it was confirmed that an enhancer RNA, a type of non-encrypted RNA, is formed in the NFATC1 during osteoblastic cell formation.
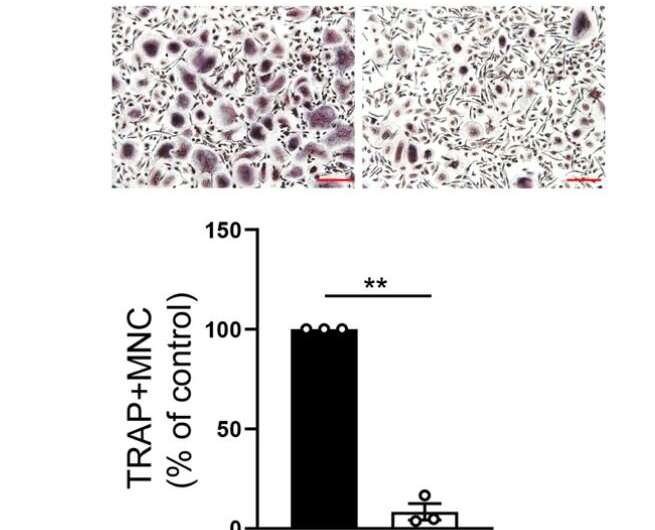
Non-encrypted RNA does not encode proteins, but plays an important role in regulating gene expression. In particular, due to the specificity of the molecular sequence, it can be easily targeted for treatment. In fact, we observed that interfering with NFATC1 super-enhancer RNA inhibits the formation of osteoblasts together.
Through this study, it has been confirmed that NFATC1 super-enhancer RNA, which is formed during osteoblast differentiation, can be used as a treatment target.
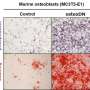
Immunoblot analysis of NFATc1 expression in human CD14+ cells transfected with control or SE-eRNA:NFATc1-specific siRNA and treated for 24 h with RANKL. α-Tubulin was the loading control. Credit: UNIST
"[O]ur study is the first to identify SEs and SE-eRNAs in human osteoclasts and provides a better understanding of human osteoclast biology, thereby opening new therapeutic avenues for human pathological bone destruction," noted the research team.
The findings of this research were made available in December 2022, ahead of its publication in the journal, Cellular and Molecular Immunology.
More information: Seyeon Bae et al, RANKL-responsive epigenetic mechanism reprograms macrophages into bone-resorbing osteoclasts, Cellular & Molecular Immunology (2022). DOI: 10.1038/s41423-022-00959-x