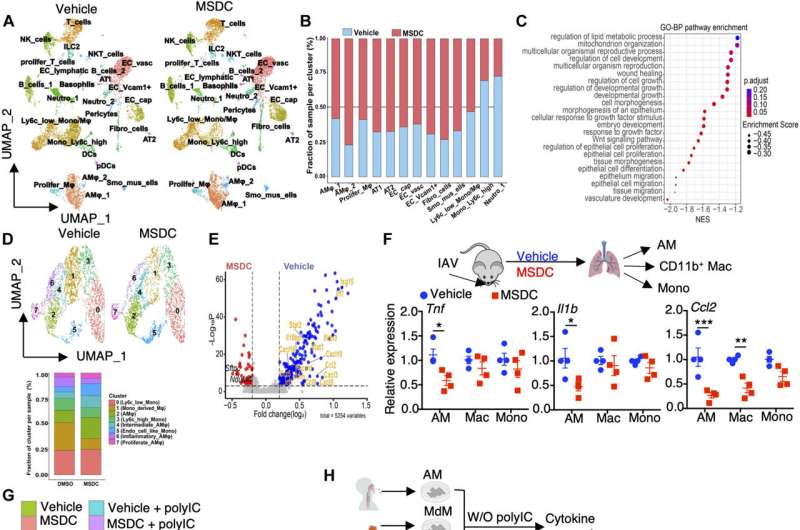
Murine and human lung macrophages are prominent target of MPC inhibition by MSDC. (A to E) scRNA-seq analysis of lungs from IAV-infected C57BL/6 WT mice with vehicle or MSDC treatment at 4 d.p.i. Lung cells were pooled from three individual mice from each group. (A) UMAP plot visualization of lung cells from vehicle- or MSDC-treated mice. (B) The relative contributions of indicated clusters by each group. (C) Dot plot showing enrichment of Gene Ontology biological processes pathways enriched in MSDC-treated lungs. The color of the dots represents the adjusted P value. Dot size represents the enrichment score. (D) UMAP showing clusters of monocytes and macrophages from (A) in vehicle- or MSDC- treated lung cells (upper panel). The percentages of each cluster in each studied subject was shown on the lower panel. (E) Volcano plot showing the differentially expressed genes in AMs (cluster 2, 4, 6 and 7) of vehicle (blue) and MSDC (red) treated mice. (F) The mRNA levels of Tnf, Il1b and Ccl2 in AMs, CD11b+ macrophages (Mac) and monocytes (Mono) sorted from lungs at 4 d.p.i. (G) RNA-seq analysis of mouse AMs stimulated with or without Poly IC in the presence of vehicle or MSDC overnight in vitro. Heatmap of K-means clustering of differentially expressed genes and KEGG enrichment analysis. (H) The mRNA levels of TNF, IL1B and CCL2 in human AMs, monocyte-derived macrophages (MdM), and monocytes (Mono) stimulated with or without Poly IC in the presence of vehicle or MSDC overnight in vitro. Data are presented as means ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001. The p value was determined by multiple t tests (F) and one-way ANOVA (H). Credit: Science Immunology (2023). DOI: 10.1126/sciimmunol.adf0348
UVA Health researchers have identified a potential treatment to prevent severe COVID-19 in patients at great risk.
The new research from the University of Virginia's Jie Sun and colleagues suggests a way to protect patients with obesity or diabetes from the runaway inflammation and dangerous blood sugar spikes that COVID-19 can cause. Such patients are at high risk for severe COVID-19 and, with the effectiveness of existing COVID treatments waning, new treatment options are needed urgently.
The study discovered a metabolic path that modulates inflammation caused by COVID-19 infections as well as promotes lung recovery in patients. The process suggests a possible treatment that could be added to existing antiviral treatments to treat severe COVID in at-risk patients, according to Sun.
"We are hoping this study could spur strong interests for clinical trials to prevent or treat severe viral infections, including COVID-19, in diabetic and/or obese individuals, using a second-generation insulin sensitizer," said Sun, of UVA's Division of Infectious Diseases and International Health and UVA's Carter Immunology Center.
Preventing severe COVID-19
Sun's approach seeks to prevent severe COVID-19 by targeting the carrier of fuel to mitochondria, the powerhouses of our cells. Sun and his team found that reducing the activity of this carrier protected obese lab mice from severe illness caused by influenza and COVID-19.
The reduction of the fuel supply simultaneously reduced harmful inflammation and promoted metabolic health. It also helped prevent harmful blood sugar spikes associated with COVID-19 and promoted lung recovery after COVID-19 and influenza pneumonia.
The researchers noted that their approach "synergized" with the antiviral component of Paxlovid, nirmatrelvir, to "markedly" reduce mortality in the lab mice. That suggests it may be possible to use an insulin-sensitizing drug to obtain similar results in human patients with COVID-19.
"We know that steroids are effective in severe COVID-19, but these drugs have side effects like elevated blood glucose that make their use more complicated in obese and diabetic patients," said study co-author Dr. Jeffrey Sturek, a UVA pulmonary and critical care physician-scientist specializing in COVID-19 disease. "The really exciting part about this pathway is the ability to treat both inflammation as well as altered glucose metabolism."
To assess the potential benefits in humans, the scientists tested their approach using human lung tissue samples. They were pleased to see the drug diminished cellular inflammation, a promising sign. More testing will be needed, however.
Based on the results, the UVA scientists and Cirius Therapeutics, the maker of the insulin-sensitizing drug the scientists used, are planning a clinical trial testing the drug in human patients with COVID-19.
Cirius Therapeutics is developing the drug to treat liver damage in patients with non-alcoholic fatty liver disease. The drug works through a recently identified mechanism to reprogram mitochondrial metabolism and has an "outstanding" safety profile, the researchers note in a scientific paper outlining their findings.
That leaves the UVA scientists hopeful that their new research could make a real difference for people at high risk for severe COVID-19.
"Diabetes increases COVID-19 severity and, conversely, COVID-19 can lead to blood glucose rise," Sun said, adding that adding the drug to the treatment regimen could ease the disease's impacts.
The researchers have published their findings in the journal Science Immunology.
More information: Bibo Zhu et al, Inhibition of the mitochondrial pyruvate carrier simultaneously mitigates hyperinflammation and hyperglycemia in COVID-19, Science Immunology (2023). DOI: 10.1126/sciimmunol.adf0348
Journal information: Science Immunology
Provided by University of Virginia