Credit: Unsplash/CC0 Public Domain
Knee and hip replacements are increasing globally due to an aging population. In the United States, the number of patients needing a joint replacement will exceed 2.7 million in the next seven years. Post-surgery infections, while rare at 1–5% of patients, result in high patient morbidity and mortality.
In the United States the annual national hospital costs for treating infection are projected to exceed $1.85 billion.
We currently use an antibiotic, cefazolin, at the time of surgery to prevent infection. But with the rise of antibiotic resistant bacteria, experts have debated whether adding a second antibiotic, vancomycin, would be better to prevent more infection. Vancomycin is a commonly used antibiotic for MRSA (methicillin resistant Staphylococcus aureus). Many centers in Australia and globally had adopted the practice of giving both cefazolin and vancomycin to prevent infections, despite the lack of clear benefit.
Now a clinical trial, published in the New England Journal of Medicine and led by Monash University researchers, in collaboration with orthopaedic surgeons and infectious diseases doctors, has found that the addition of vancomycin did not protect against infection and may have led to more infections and more adverse reactions for the patients.
According to the study lead, Professor Trisha Peel, from the Monash University Central Clinical School, "Given the number of joint replacements performed in Australia and globally, our trial has answered the important about whether more antibiotics are better for our patients having joint replacement surgery: with the definitive answer being 'no.' This trial will have a significant impact on practice," she said.
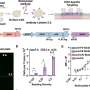
The Australian Surgical Antibiotic Prophylaxis (ASAP) trial looked at 4,239 patients without a history of MRSA, in 11 hospitals across Australia, including regional and private hospitals.
Patients were randomized to receive receive either vancomycin or saline placebo, in combination with cefazolin. Among all patients, the addition of vancomycin was no better than the traditional cefazolin antibiotic.
Unexpectedly, in patients undergoing knee joint replacement, the risk of infection was higher in the vancomycin group, 5.7%, than in the placebo group, with 3.7% infection rate.
Professor Peel said that the study reflects how important these large, randomized, multi-center clinical trials are "A lot of things seem to make sense, but we don't really know for sure until they are tested in a clinical trial."
"This is one of those cases—more antibiotics weren't better, and in some people might have actually been worse." she said.
More information: Trial of Vancomycin and Cefazolin as Surgical Prophylaxis in Arthroplasty, New England Journal of Medicine (2023). DOI: 10.1056/NEJMoa2301401
Journal information: New England Journal of Medicine
Provided by Monash University