The adjuvanted recombinant nanoparticle-based vaccine GBP510/AS03 shows a clinically acceptable safety profile and immunogenicity results comparable to that of the currently available ChAdOx1-S vaccine. Credit: Cheong et al., Korea University
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019. Since then, extensive efforts have been made to develop and evaluate vaccines to combat the virus. One of the promising candidates is GBP510 a recombinant vaccine adjuvanted with AS03, designed to target the SARS-CoV-2 virus's spike receptor-binding domains.
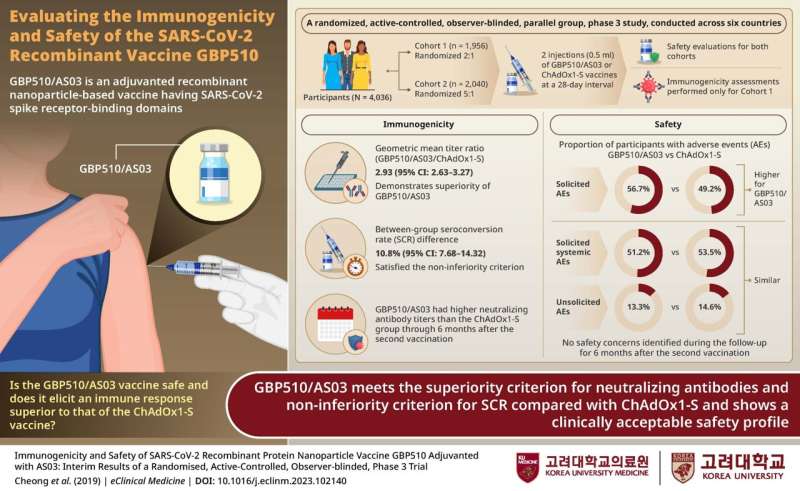
A team of researchers, led by Professor Hee Jin Cheong and Joon Young Song from the Korea University College of Medicine in Seoul, conducted a randomized, active-controlled, observer-blinded, parallel-group, phase 3 study across six countries, to assess the immunogenicity and safety of GBP510/AS03, relative to the ChAdOx1-S vaccine.
Their research was published in eClinical Medicine. "In the prevailing context where mRNA vaccines have taken the center stage, we examined the outcomes of a preceding phase 1/2 study, that demonstrated the high immunogenicity of the adjuvanted nanoparticle-based vaccine platform GBP510/AS03. This led us to embark on the current study," claimed Professor Cheong.
The participants were split into two groups: those with no prior SARS-CoV-2 infection or COVID-19 vaccination history and those without these parameters. Each participant based on randomization, received two injections of either GBP510/AS03 or ChAdOx1-S spaced 28 days apart.
The participants from the first group underwent immunogenicity analysis measuring the neutralizing antibody response. The researchers found that the immune response induced by two doses of GBP510/AS03 was superior to that caused by ChAdOx1-S.
The higher neutralizing antibodies with GBP510/AS03 were seen in participants regardless of age, sex, or ethnicity. Regarding safety assessment, GBP510/AS03 demonstrated a clinically acceptable safety profile; no safety concerns were identified throughout the study period.
"The incorporation of an adjuvant into the nanoparticle-based vaccine platform has showcased both a robust ability to enhance the immune response and safety in this extensive phase 3 clinical trial. This development opens up the potential for broad future applications, not only in the creation of new infectious disease vaccines but also in the enhancement of existing vaccines to elevate their safety and efficacy," said Prof. Song.
More information: Joon Young Song et al, Immunogenicity and safety of SARS-CoV-2 recombinant protein nanoparticle vaccine GBP510 adjuvanted with AS03: interim results of a randomised, active-controlled, observer-blinded, phase 3 trial, eClinicalMedicine (2023). DOI: 10.1016/j.eclinm.2023.102140
Journal information: EClinicalMedicine
Provided by Korea University College of Medicine