Phase 3 clinical trial: Second-generation COVID vaccine generates high levels of neutralizing antibodies
A COVID-19 vaccine developed at the University of Washington School of Medicine has proven safe and effective in late-stage clinical testing. SK bioscience, the company leading the vaccine's clinical development, will seek authorization for its use in South Korea within the month.
The Seattle scientists behind the new vaccine sought to create a "second-generation" vaccine for COVID-19 that is safe, effective at low doses, simple to manufacture at scale, and stable without deep freezing. These attributes would enable vaccination at a global scale by reaching people in areas where medical, transportation, and storage resources are limited.
If GPB510 receives full approval from regulators, it will be made available through COVAX, an international effort working to equitably distribute COVID vaccines around the world. In addition, the South Korean government has entered a purchase agreement to secure 10 million doses for domestic use.
The University of Washington is licensing the vaccine technology royalty-free for the duration of the pandemic.
A multinational Phase 3 trial involving 4,037 adults over 18 years of age found that the vaccine, dubbed GPB510, elicits higher levels of protective antibodies than the Oxford/AstraZeneca vaccine Vaxzevria. In these studies, GPB510 or Vaxzevria was administered twice with an interval of 4 weeks.
In addition, the "antibody conversion rate," which refers to the proportion of subjects whose neutralizing antibody level increased fourfold or more after vaccination, was higher with GPB510.
T cell activation levels, which protect the body from COVID-19, were also similar or higher with GBP510.
Phase 1/2 trial results announced by SK bioscience this past November and posted as a preprint in March found that GPB510 was safe and produced virus-neutralizing antibodies in all trial participants receiving the adjuvanted vaccine. Today, April 25, SK bioscience issued a press release on its Phase 3 trial findings: SK bioscience Reports Positive Phase 3 Immunogenicity Results of Its Adjuvanted COVID-19 Vaccine Clinical Trial of COVID-19 Vaccine, AS03-adjuvanted.
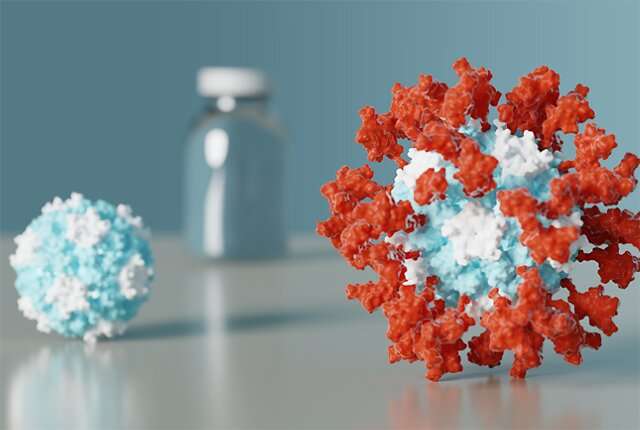
Unlike the earliest approved vaccines for COVID-19 that make use of mRNA, viral vectors, or an inactivated virus, GPB510 is made of proteins that form tiny particles studded with fragments of the pandemic coronavirus. These nanoparticles were designed by scientists at UW Medicine and advanced into clinical trials by SK bioscience and GlaxoSmithKline with financial support from the Coalition for Epidemic Preparedness Innovations, also known as CEPI. GPB510 includes GSK's pandemic adjuvant, AS03.
"This vaccine was designed at the molecular level to present the immune system with a key part of the coronavirus spike protein. We know this part, called the receptor-binding domain, is targeted by the most potent antibodies," said Neil King, assistant professor of biochemistry at the University of Washington School of Medicine.
Two laboratories in the UW School of Medicine Department of Biochemistry led the initial development of the protein-based vaccine: the King Lab pioneered the vaccine's self-assembling protein nanoparticle technology while the Veesler Lab identified and integrated a key fragment of the SARS-CoV-2 Spike protein onto the nanoparticles.
David Veesler, an associate professor of biochemistry and HHMI investigator at UW Medicine, has been studying coronaviruses since 2015. Using advanced electron microscopes, researchers in the Vessler lab were the first to identify how the novel coronavirus enters human cells. They were also among the first to report, in Cell, detailed structural information about the virus' spike protein, a critical piece of its infectious machinery.
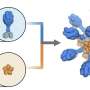
In 2016, scientists in the King lab at the UW School of Medicine began developing a strategy for building a new type of vaccine. They designed proteins that self-assemble into precise spherical particles and later showed that these nanoparticles could be decorated with proteins from a virus.
Researchers from the two labs worked together in the earliest months of the COVID-19 pandemic to design a protein nanoparticle decorated with 60 copies of the Spike protein receptor-binding domain. The designed nanostructure mimics the repetitive nature of proteins on the surface of viruses, a property that the immune system responds strongly to.
"In order to focus the antibody response where it matters most, we decided to include in the vaccine only a key fragment of the coronavirus spike protein, known as the receptor-binding domain," said Veesler. "We are thrilled to see that this strategy paid off and has led to a successful subunit vaccine."
In initial animal studies reported in late 2020 in Cell, the nanoparticle vaccine was found to produce high levels of virus-neutralizing antibodies at low doses. These antibodies target several different sites on the coronavirus Spike protein, a desirable quality that may enhance protection against future coronavirus variants.
Further preclinical studies, published in Nature, also showed that the vaccine conferred robust protection and produced a strong B-cell response in non-human primates, which may improve how long the protective effects of the vaccine last.
In a recent preprint, a third dose of the vaccine was found to confer strong protection against the omicron variant of COVID-19 in animals. SK bioscience will initiate testing third doses in 750 human adults soon.
More information: SK bioscience Reports Positive Phase 3 Immunogenicity Results of Its Adjuvanted Covid-19 Vaccine Clinical Trial of COVID-19 Vaccine, AS03-adjuvanted