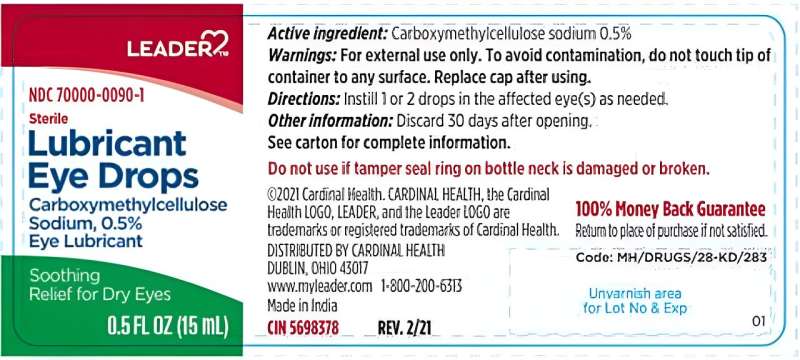
Cardinal Health is voluntarily recalling certain LEADER brand eye drops because they may cause eye infections.
The U.S. Food and Drug Administration informed the Dublin, Ohio, company that agency investigators found unsanitary conditions in its manufacturing facility. Tests of critical drug production areas of the facility were positive for bacteria. The drops were supplied by Velocity Pharma.
Cardinal Health has received reports of three adverse events related to these products and have shared this information with its supplier, the recall notice said.
These eye drops are sold over the counter for temporary relief of burning and irritation due to dryness, to protect against further irritation, and to relieve redness. They were sent to wholesalers and retailers starting in December 2021.
While the company is notifying its direct accounts via mail and arranging for return of the products, wholesalers, distributors, and retailers should stop selling the eye drops.
Consumers should also stop using them. They may return any of the recalled products to the place they bought them.
More information: More Information
Copyright © 2023 HealthDay. All rights reserved.