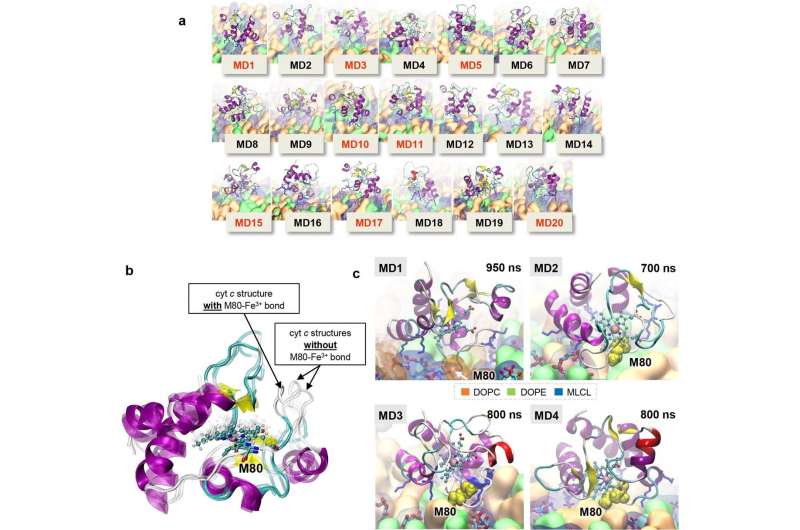
Final shapshots from simulations of cyt c – membrane interactions and superposition of the conformations of cyt c reached after 100 ns runs. Credit: Nature Metabolism (2023). DOI: 10.1038/s42255-023-00926-4
In a Nature Metabolism paper, researchers from the University of Pittsburgh detail a potential new target and a small-molecule drug candidate for treating Barth syndrome, a rare, life-threatening and currently incurable genetic disease with devastating consequences.
Barth syndrome affects about 1 in every 300,000 to 400,000 babies born worldwide. Those with the condition have weak muscles and hearts and experience debilitating fatigue and recurrent infections.
Pitt researchers discovered that faulty mitochondria are at least partially to blame, and identified a molecular culprit that could be targeted to potentially reverse the disease course in the future.
In healthy people, a lipid known as cardiolipin, or CL, undergoes a series of transformations, known as remodeling, within the mitochondria, explains senior author of the study Dr. Valerian Kagan, professor of environmental and occupational health at the University of Pittsburgh School of Public Health.
In people with Barth syndrome, however, a crucial mitochondria-housed gene, called tafazzin (TAZ), is mutated. Without TAZ, CL remodeling is stopped in its tracks and harmful lipids accumulate.
For this latest investigation, the team used computational models as well as in-vitro studies in both mouse myoblast cells and human heart-tissue samples from people with Barth syndrome.
"We found that lyso-cardiolipin, an intermediate accumulating in mutant TAZ-deficient cells, interacts with the mitochondrial protein cytochrome c, converting it to a demon enzyme that oxidizes everything around it," said Kagan.
As it turns out, this excessive oxidation in TAZ-deficient cells could be prevented. The team showed that a compound known as imidazole-substituted oleic acid, or IOA, could block the formation of those complexes and improve the motor function and endurance in a fruit fly model of Barth syndrome.
In the future, this discovery could pave way for correcting genetic tafazzin deficiency and improving mitochondrial function through small-molecule therapeutics.
More information: Anomalous peroxidase activity of cytochrome c is the primary pathogenic target in Barth syndrome, Nature Metabolism (2023). DOI: 10.1038/s42255-023-00926-4
Journal information: Nature Metabolism
Provided by University of Pittsburgh