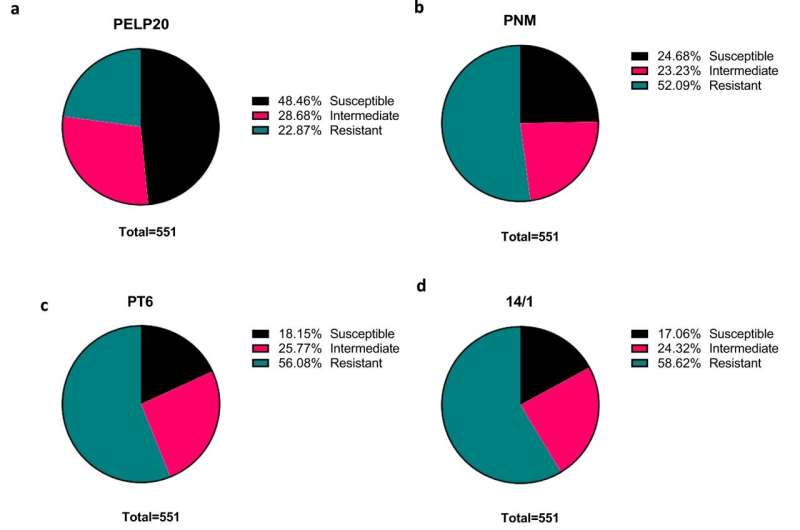
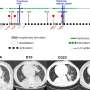
Phage cocktail screening against 551 clinical isolates. Percentage of isolates susceptible (black—complete lysis), intermediate (pink—incomplete lysis) and resistant (green—no lysis) determined by direct spot test method for phages a) PELP20 b) PNM c) PT6 d) 14/1 e) cocktail and f) Frequency of phage susceptibility across clinical isolates measured as number of phages each isolate shows susceptible to. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-45785-z
A new study describes the use of phage therapy to eradicate multi-drug resistant Pseudomonas aeruginosa infections in a living organism (in vivo) with important new implications for antibiotic resistance.
Research published in Nature Communications by University of Liverpool investigators shows important new findings in the fight against the World Health Organization's (WHO) priority pathogen.
Antimicrobial resistance (AMR) poses a serious global threat to our ability to treat bacterial infections. One such pathogen is Pseudomonas aeruginosa, a major hospital-acquired infection that causes severe disease including sepsis. Antibiotic resistant P. aeruginosa is recognized by the WHO as a priority 1 pathogen, with urgent need for new therapeutics. As such, there is renewed interest in using bacteriophages (phages) as a therapeutic.
Phages—viruses that "eat" bacteria—can be used to treat bacterial infections and reduce resistance to antibiotics by removing barriers—known as biofilms—which reduce antimicrobial effectiveness. Each phage can target individual bacteria, can be combined with multiple phages and antibiotics, and adapted specifically for each individual patient.
The dynamics of treating P. aeruginosa with phage in vivo are poorly understood. However, a new paper illustrates potentially game-changing possibilities in using phage therapy to reduce the bacterial burden of multi-drug resistant P. aeruginosa and simultaneously re-sensitize these bacteria to antibiotics.
The lead investigators, Professor Jo Fothergill, and Professor Aras Kadioglu from the Department of Clinical Infection, Microbiology & Immunology at the University of Liverpool said, "We developed a new model to investigate pan-resistant P. aeruginosa systemic infections in vivo. Using this model, we showed that phage therapy displays strong therapeutic potential, clearing infection from multiple organ sites and blood.
"Some remaining bacteria, particularly in the lungs, displays phage resistance due to limiting phage adsorption, amazingly however, resistance to phage results in re-sensitization to a wide range of antibiotics eventually leading to bacterial clearance.
"The potential to use phage therapy to reduce the bacterial burden of pan-resistant P. aeruginosa and simultaneously re-sensitize bacteria to antibiotics is game changing. Therefore, sequential administration of bacteriophage and antibiotic therapy could be a viable solution in combating pan-resistant P. aeruginosa infections."
More information: Eleri A. Ashworth et al, Exploiting lung adaptation and phage steering to clear pan-resistant Pseudomonas aeruginosa infections in vivo, Nature Communications (2024). DOI: 10.1038/s41467-024-45785-z
Journal information: Nature Communications
Provided by University of Liverpool