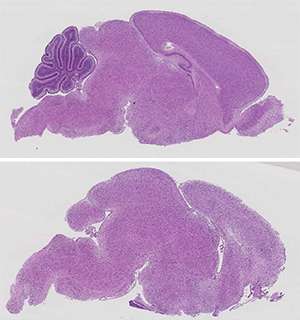
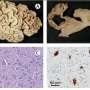
Relative to a normal brain (top), the brains of mice lacking the Prmt5 gene are underdeveloped and lack key structural components (bottom). Credit: M. Bezzi, et al.
A newly discovered early-warning system triggers cellular suicide when a critical RNA editing system breaks down.
The initial RNA transcript generated from a given gene is only a first draft that must be heavily edited before it is translated. This process entails splicing together segments of the RNA—known as 'exons'—that actually encode a protein product, while removing the noncoding 'intron' sequences that lie between the exons. Improper splicing results in proteins that are defective or entirely nonfunctional. Now, research from Ernesto Guccione's team at the A*STAR Institute of Molecular and Cell Biology in Singapore has uncovered a splicing 'quality-control system' that could also offer a mechanism for fighting cancer.
Guccione and co-workers began by studying the function of protein arginine methyltransferase 5 (PRMT5), an enzyme that introduces chemical modifications onto other proteins. Because PRMT5 is known to act within the brain, the researchers generated transgenic mice that no longer produced this protein in their central nervous system. These animals died within weeks of birth, with profound neurological defects and apparent disruption of brain development.
Careful examination of cultured neuronal precursor cells showed that the absence of PRMT5 triggered a cellular 'self-destruct' mechanism known as apoptosis. This process was mediated in part by p53, a protein that acts as a critical safeguard against cancer by triggering apoptosis in cells on the verge of tumor formation.
Several components of the cellular splicing machinery are among PRMT5's targets. Guccione and co-workers determined that the increased apoptosis observed in the absence of PRMT5 was a direct result of splicing defects. Without the chemical modifications introduced by PRMT5, the splicing proteins failed to assemble properly. The researchers identified hundreds of genes from PRMT5-deficient cells in which introns had been retained or exons had been inappropriately eliminated.
Among the affected proteins was MDM4, a protein that inhibits p53, thus preventing apoptosis and facilitating tumor formation. In the absence of PRMT5, the RNA encoding MDM4 loses an exon, yielding a truncated and unstable protein product that is no longer able to block p53-mediated apoptosis.
This vulnerability to mis-splicing turns Mdm4 into an important quality-control system. "We have identified a 'sensing mechanism' that directly links defects in the splicing machinery to activation of the p53 pathway," says Guccione. This mechanism is also present in human cells. His group sees great potential in deliberately tripping the MDM4 sensor system as a means to selectively kill cancer cells. "We have already obtained very promising initial results both in cell lines and in mouse models," he says.
More information: Bezzi, M., Teo, S. X., Muller, J., Mok, W. C., Sahu, S. K. et al. Regulation of constitutive and alternative splicing by PRMT5 reveals a role for Mdm4 pre-mRNA in sensing defects in the spliceosomal machinery. Genes & Development 27, 1903–1916 (2013).
Journal information: Genes & Development