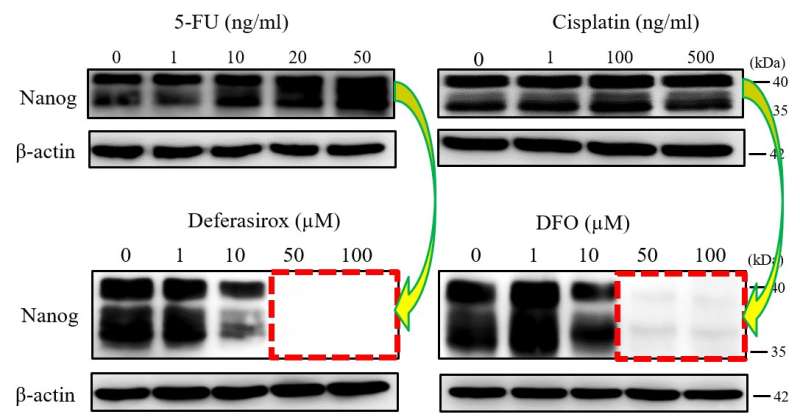
Iron chelators Deferasirox and Deferoxamine (DFO) suppress the expression of stemness marker. 5-FU, ordinary anti-cancer drug, did not inhibited. Credit: Okayama University
Researchers at Okayama University report in Oncotarget a promising method for targeting cancer stem cells that cause tumor growth and cancer relapse. The approach involves administering molecules that capture iron, an overload of which is known to be a potential cause of cancer.
Cancer stem cells (CSCs) are similar to normal stem cells in that they can differentiate—become cells of a specialized type. CSCs are resistant to radio- and chemotherapy and are believed to play an important role in metastasis and cancer relapse. Since iron overload is known to cause certain types of cancer, Dr. Toshiaki Ohara and Dr. Takayuki Ninomiya from Okayama University and colleagues have now tested whether the suppressing of iron levels can halt proliferation and 'stemness' of CSCs. The researchers found that controlled iron depletion indeed has the potential to become a therapy for targeting CSCs.
Dr. Ohara and colleagues first confirmed that in vitro, a supply of iron leads to the proliferation of CSCs, as well as the persistence of their stemness. Then, the researchers studied the effect of administering iron chelators: organic compounds that bond to particular metals, in this case iron. The scientists reckoned that through chelation, iron atoms are isolated and, potentially, 'neutralized'. Two known iron chelators were tested: deferasirox and deferoxamine. Both compounds suppressed the proliferation of a set of mouse cancer cells. The associated fibroblasts (cells that play a key role in cancer progression and believed to originate from CSCs), however, were not affected.
Regarding the mechanism causing the suppression of CSC proliferation, Dr. Ohara and colleagues found that the iron chelators induced apoptosis (a highly regulated process leading to cell death). The scientists also studied the effect of deferasirox and deferoxamine on the CSCs' stemness, and observed that the expression of stemness markers was suppressed—indirect evidence for stemness loss. The latter property was not observed in chemotherapy treatment, indicating the unique function of iron chelators as stemness marker suppressors. Finally, the researchers tested the effect of deferasirox and deferoxamine in vivo; both iron chelators suppressed CSC tumor growth and stemness marker expression.
The results of Dr. Ohara and colleagues show that the use of iron chelators could develop into an effective cancer treatment, complementary to standard chemotherapy. In the words of the researchers: "Although … the mechanisms [of stemness marker expression] are currently unknown … this study may represent an important first step in the development of novel iron chelation based strategies for CSC therapy."
Cancer stem cells
A cancer stem cell (CSC) is a cancerous cell with the property of a normal stem cell that it can differentiate in various cell types found in a particular cancer sample. CSCs are tumor-forming, and cancer therapies targeting them are being developed, as CSCs are involved in cancer metastasis and tumor relapse.
Dr. Ohara from Okayama University and colleagues have now shown that controlled iron depletion in tumors is a promising novel strategy for targeting CSCs.
Chelation
Chelation is a type of bond between metal ions and molecules or molecular ions. It involves a multiple-bonded ligand binding to the metal ion; the ligand is usually an organic compound and called a chelant or chelator.
Since iron overload in the human body can lead to the formation of free radicals, which in turn can cause cancer, Dr. Ohara and colleagues investigated whether iron depletion through chelation, using the iron chelators deferasirox and deferoxamine, can suppress the proliferation and stemness of cancer stem cells.
More information: Takayuki Ninomiya et al. Iron depletion is a novel therapeutic strategy to target cancer stem cells, Oncotarget (2017). DOI: 10.18632/oncotarget.21846
Journal information: Oncotarget
Provided by Okayama University