Targeting bladder cancer's Achilles heel: stem cells
Two different proteins work separately as well as synergistically to feed a small pool of stem cells that help bladder cancer resist chemotherapy, research led by a Johns Hopkins Kimmel Cancer Center scientist suggests. The finding, published online in Cancer Research, could lead to new targets to fight this deadly disease and potentially other cancers as well.
Urothelial carcinoma of the bladder (UCB) is the most common cancer of the urinary tract. Just in the U.S., tens of thousands of patients are diagnosed with this disease every year, which kills more than 100,000 people worldwide annually.
One reason that bladder cancer is so deadly is the propensity for these tumors to develop resistance to the drugs typically used as frontline therapies, explains study leader Mohammad Hoque, D.D.S, Ph.D., an associate professor of otolaryngology-head and neck surgery, urology, oncology and member of the Johns Hopkins Greenberg Bladder Cancer Institute. Recent research suggests that this resistance is caused by a small pool of cancer stem cells (CSCs) within these tumors that isn't killed with chemotherapies, leading tumors to regrow and spread even after initial treatment success.
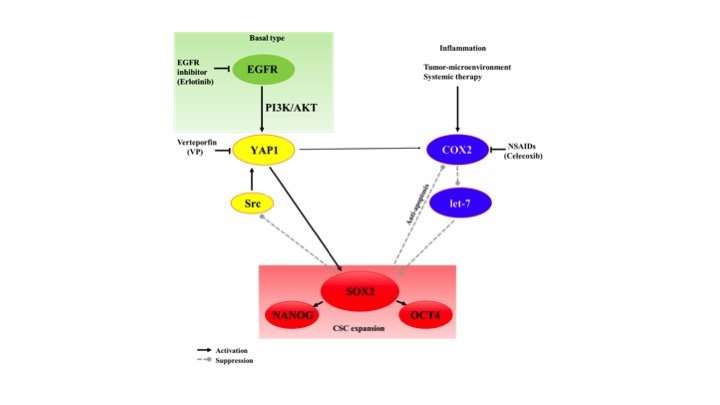
Although eliminating these CSCs is critical to fighting bladder cancer, Hoque adds, little is known about the mechanisms behind how tumors maintain their stem cell populations. To investigate, Hoque and his team, including collaborators from the Allegheny Health Network Cancer Institute, examined the roles of different proteins that had already been identified as being associated with CSCs' unique cancer-driving traits: Yes-associated protein1 (YAP1) and cyclooxygenase 2 (COX2).
Working with CSCs from human UCB tissue samples in petri dishes, the researchers used a drug known as celecoxib or genetic techniques to decrease the amount of COX2 that these cells produced. Their results showed that when COX2 expression went down, the amount of SOX2—a protein that other experiments showed was pivotal to the CSC's ability to self-renew, migrate, and invade surrounding tissue—also decreased somewhat. Similarly, inhibiting YAP1 with a drug known as verteporfin or with genetic techniques also led to a modest decrease in SOX2.
However, when the researchers inhibited production of both COX2 and YAP1 at the same time, SOX2 expression was dramatically inhibited, leading to a severe reduction of the ability of these cells to grow into tumors. The findings suggest that COX2 and YAP1 work both independently and together to regulate CSC activity through SOX2.
"Thus, targeting COX2 and YAP1 together may be indispensable for eradicating CSCs," Hoque says.
Indeed, using a different model in which UCB CSCs were grafted into mice and allowed to grow into tumors, the researchers found that inhibiting either COX2 or YAP1 with drugs enhanced the tumors' response to gemcitabine and cisplatin, a combination chemotherapy regimen often used to treat UCB. But using both inhibiting drugs together with the combination chemotherapy led to an even more dramatic response, causing the animals' tumors to continuously regress during the entire treatment cycle.
Further experiments suggested that encouraging UCB CSCs to overexpress COX2 and YAP1, as patients' tumor samples tend to do, led the cells to become resistant to drugs that target a cancer-related protein known as the epidermal growth factor receptor (EGFR). When the researchers treated the tumor-engrafted mice with a drug known as erlotinib that targets EGFR, along with the combination chemotherapy treatment and COX2 and YAP1 inhibitors, they found the most dramatic tumor regression of all, even in an aggressive subcategory of UCB known as basal-type.
Together, Hoque says, these results suggest that COX2 and YAP1 work individually and together to regulate CSCs in UCB. Targeting both proteins jointly could help improve the response of tumors to standard chemotherapy regimens and avoid chemotherapy resistance.
"Because the drugs that inhibit these proteins are already FDA approved to treat other conditions," Hoque adds, "it sets the stage for an easy transition to clinical trials."
More information: Cancer Research (2018). DOI: 10.1158/0008-5472