Custom-built high-speed real-time intravital microscope platform. Credit: KAIST
A KAIST research team succeeded in visualizing pulmonary microcirculation and circulating cells in vivo with a custom-built 3-D intravital lung microscopic imaging system. They found a type of leukocyte called a neutrophil aggregate inside the capillaries during sepsis-induced acute lung injury (ALI), leading to disturbances and dead space in blood microcirculation.
According to the researchers, this phenomenon is responsible for tissue hypoxia causing lung damage in the sepsis model, and mitigating neutrophils improves microcirculation as well as hypoxia.
The lungs are responsible for exchanging oxygen with carbon dioxide gases during the breathing process, providing an essential function for sustaining life. This gas exchange occurs in the alveoli, each surrounded by many capillaries containing the circulating red blood cells.
Researchers have been making efforts to observe microcirculation in alveoli, but it has been technically challenging to capture high-resolution images of capillaries and red blood cells inside the lungs that are in constant breathing motion.
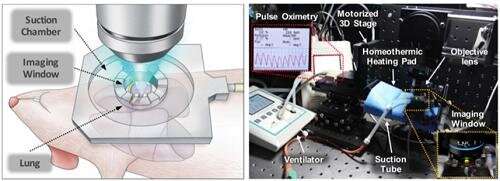
Professor Pilhan Kim from the Graduate School of Medical Science and Engineering and his team developed an ultra-fast laser scanning confocal microscope and an imaging chamber that could minimize the movement of a lung while preserving its respiratory state. They used this technology to successfully capture red blood cell circulation inside the capillaries of animal models with sepsis.
Illustrative schematic and photo of a 3D intravital lung microscopic imaging system. Credit: KAIST
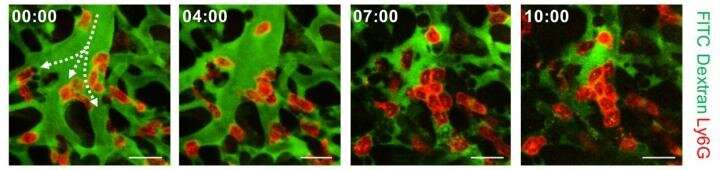
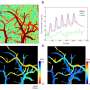
During the process, they found that hypoxia was induced by the increase of dead space inside the lungs of a sepsis model, a space where red blood cells do not circulate. This phenomenon is due to the neutrophils aggregating and trapping inside the capillaries and the arterioles. It was also shown that trapped neutrophils damage the lung tissue in the sepsis model by inhibiting microcirculation as well as releasing reactive oxygen species.
Further studies showed that the aggregated neutrophils inside pulmonary vessels exhibit a higher expression of the Mac-1 receptor (CD11b/CD18), which is a receptor involved in intercellular adhesion, compared to the neutrophils that normally circulate. Additionally, they confirmed that Mac-1 inhibitors can improve inhibited microcirculation, ameliorate hypoxia, while reducing pulmonary edema in the sepsis model.
Their high-resolution 3-D intravital microscope technology allows the real-time imaging of living cells inside the lungs. This work is expected to be used in research on various lung diseases, including sepsis.
Aggregation of neutrophils and consequent flow disturbance in pulmonary arteriole in sepsis-induced lung injury. Credit: KAIST
The research team's pulmonary circulation imaging and precise analytical techniques will be used as the base technology for developing new diagnostic technologies, evaluating new therapeutic agents for various diseases related to microcirculation.
Professor Kim said, "In the ALI model, the inhibition of pulmonary microcirculation occurs due to neutrophils. By controlling this effect and improving microcirculation, it is possible to eliminate hypoxia and pulmonary edema—a new, effective strategy for treating patients with sepsis."
More information: Inwon Park et al, Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury, European Respiratory Journal (2019). DOI: 10.1183/13993003.00786-2018
Journal information: European Respiratory Journal