Pampee Young, MD, PhD, left, Sarika Saraswati, PhD, and colleagues are studying the different ways fibroblasts function following tissue injury. Credit: Joe Howell
Following tissue injury, fibroblast cells activate, divide and play key roles in both tissue repair and pathological scarring—fibrosis—that can drive organ failure.
Vanderbilt investigators have now discovered that, in contrast to prevailing dogma, fibroblasts are not all alike; instead, they have distinctive functions following tissue injury.
"Our work offers a new perspective over the currently held thinking that fibroblasts are a single population of cells working in the same manner to coordinate wound repair," said Pampee Young, MD, Ph.D., adjunct professor of Pathology, Microbiology and Immunology.
The findings, reported in Nature Communications, suggest that it might be possible to prevent the pathological scarring effects of fibroblasts without impairing the functions that are necessary for wound healing.
Young, who is the Chief Medical Officer of the American Red Cross, and Sarika Saraswati, Ph.D., research assistant professor of Pathology, Microbiology and Immunology, have focused on understanding how organs repair themselves after injury.
They knew that although activated fibroblasts appear to be heterogenous, most studies of functional roles have treated the cells as a homogenous entity.
"Our goal was to identify and understand functional differences in major post-injury fibroblast subtypes," Saraswati said.
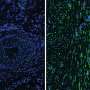
The researchers evaluated the expression patterns of two marker proteins expressed in injury-activated fibroblasts: fibroblast specific protein 1 (FSP1) and alpha smooth muscle actin (alphaSMA) in mouse models of heart, skin and kidney injury, and in human heart tissue collected after heart attacks.
They found that FSP1 and alphaSMA were expressed by distinct fibroblast cells after tissue injury and that FSP1 fibroblasts were present at wound sites earlier than alphaSMA fibroblasts. Previous studies have characterized alphaSMA as a hallmark of pathological fibrosis.
To explore molecular and functional features of these fibroblast subtypes, the researchers isolated FSP1 and alphaSMA cells from mouse models of heart injury.
They found that the FSP1 fibroblasts had pro-angiogenic (blood vessel promoting) gene expression and protein profiles. In an in vivo wound healing assay, the FSP1 cells promoted blood vessel development.
Together, the gene signature and functional findings support a role for FSP1 fibroblasts in immune cell recruitment to wound sites, cellular proliferation and angiogenesis.
These characteristics distinguish the FSP1 fibrolast subtype from the pro-fibrotic alphaSMA fibroblast, the researchers noted.
"This study clarifies the molecular and functional uniqueness of two distinct and prevalent post-injury fibroblast subtypes and begins to fill a critical gap in our knowledge about the roles of fibroblasts in healing and fibrosis," Saraswati said.
"We hope that greater understanding of fibroblast subtypes will provide a new paradigm for treating organ fibrosis."
More information: Sarika Saraswati et al. Identification of a pro-angiogenic functional role for FSP1-positive fibroblast subtype in wound healing, Nature Communications (2019). DOI: 10.1038/s41467-019-10965-9
Journal information: Nature Communications
Provided by Vanderbilt University