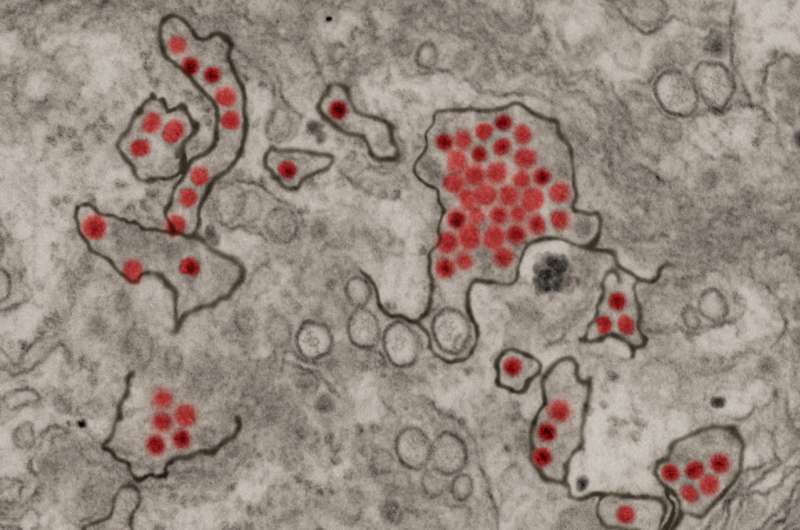
Zika virus particles (red) shown in African green monkey kidney cells. Credit: NIAID
Amyloids are particular protein assemblies with properties similar to silk, that serve numerous functions. They also form upon protein misfolding resulting in protein inactivation.
Frederic Rousseau and Joost Schymkowitz (VIB-KU Leuven) used these properties to invent synthetic amyloid peptides that can be tailored to switch-off the function of desired target proteins. These peptides, termed Pept-ins, already proved to be a valuable approach to tackle bacterial pathogens or slow down tumor growth. Now, Schymkowitz and Rousseau's team wanted to explore whether pept-ins could also be used to inactivate viral proteins and thereby interfere with viral replication.
The researchers designed two Pept-ins encoding virus-specific amyloid sequences identified in influenza A and Zika virus proteins, respectively. In collaboration with Xavier Saelens (VIB-UGent) and Johan Neyts (KU Leuven), they tested the antiviral properties of these molecules.
"We found that each amyloid interferes with the replication of the corresponding virus," explains Emiel Michiels, Ph.D. student in the lab of Schymkowitz and Rousseau. The effects turned out to be specific, adds Michiels: "For influenza A, we show that our synthetic amyloid accumulates at the site of infection and interferes with viral replication in mice. The amyloid binds to the viral target protein, forcing the protein into a non-functional conformation. Influenza B is not affected by this Pept-in, highlighting the sequence specificity of this interaction."
The new antiviral applications broaden the therapeutic potential of the Pept-in technology platform, which is explored by Aelin Therapeutics—a spin-off company based on Schymkowitz and Rousseau's research. The researchers hope to investigate whether the same approach also work to target other types of viruses.
More information: Emiel Michiels et al. Reverse engineering synthetic antiviral amyloids, Nature Communications (2020). DOI: 10.1038/s41467-020-16721-8
Journal information: Nature Communications
Provided by VIB (the Flanders Institute for Biotechnology)