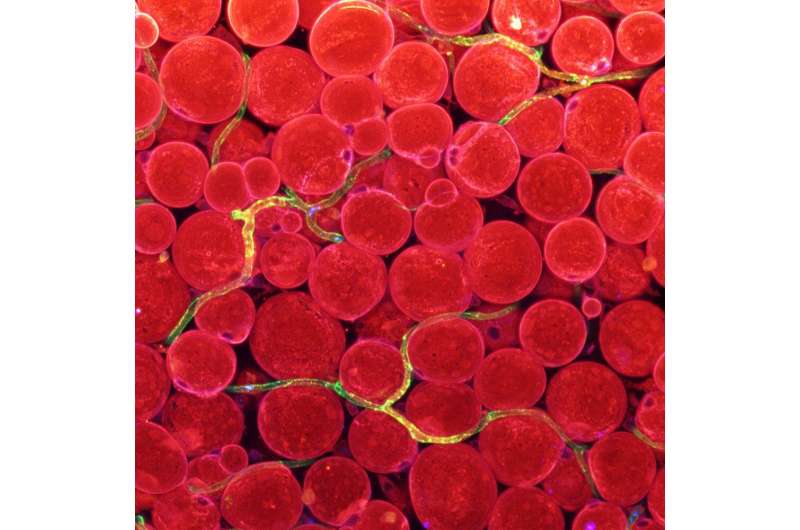
Figure 1. Expression of fatty acid transporters in blood vessels. Fatty acid transporters (CD36; blue) in blood vessels (green), whose overlapping shown in yellow green, transverse around fat cells (red) under the skin. Credit: Institute for Basic Science
Can obesity define health? It is a question for much debate. Still, obesity is generally classified into metabolically healthy obesity (MHO) and an unhealthy version of obesity. As we grow older, we tend to put on excess fat more around the waist than the hips and legs with aging, becoming more "apple-shaped" than "pear-shaped" and also at a greater risk of metabolic syndrome. As fat accumulates around our abdominal organs, instead of under the skin where most of our body fat usually sits, this visceral fat releases fatty acids and inflammatory substances directly into the liver, causing toxicity and insulin resistance. People with MHO, meanwhile are characterized by favorable health parameters, including high insulin sensitivity, no signs of hypertension, and less inflammation, and a healthier immune system.
It is undeniable that where body fat sits matters for health, but little has been known about what mechanism determines whether we have the pear shape or the apple shape. Led by Dr. KOH Gou Young at the Center for Vascular Research, within the Institute for Basic Science (IBS), South Korea, scientists have reported Angiopoietin-2 (Angpt2) - a hormone secreted from fat tissue, previously known as a protein coding peptide involved in embryonic vascular development—as a key driver that inhibits the accumulation of potbellies by enabling the proper transport of fatty acid into general circulation in blood vessels, thus preventing insulin resistance. Their findings have been published online in the journal Nature Communications (12 June 2020).
"Previous approaches blocked the expression of Angpt2 in the whole circulatory system with pharmacological interventions to inhibit displaced fat accumulation that leads to metabolic disorders. Though the vascular endothelial cell is the anatomic and metabolic gatekeeper of fat shuttling into tissues, it has still remained uncertain whether the displaced accumulation of excess fat in fat tissues is a cause or an indicator of metabolic syndrome. We specifically focused on the fatty acid shuttling in the specific fat tissues under the skin. Angpt2 is found to play a key role in packing on fat on proper locations where they should be to contain wider waistline," says BAE Hosung, the first author of this study.
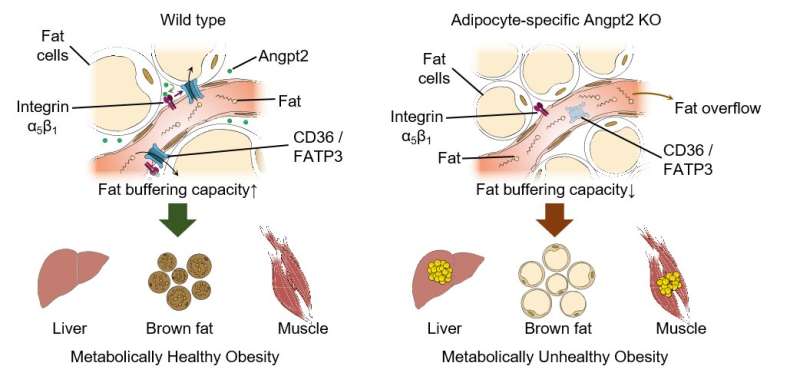
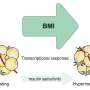
Figure 2. Endothelial-to-adipocyte fatty acid transport determines metabolic health. Angpt2 produced from fat cells interacts with its receptor integrin α5β1 to drive fatty acid transporters (CD36 and FATP3) and ensures normal circulating fat. If this Angpt2–integrin α5β1 signaling does not work, defects in buffering capacity of fat tissue lead to fat spillover into the liver and muscle, convert brown fat to white fat, and consequently result in defects in metabolic health.
Noting that the blood vessels under the skin are home to certain fatty acid transport proteins, the researchers compared samples from MHO and metabolically unhealthy obese individuals. Angpt2 turned out to be the single potential candidate for sustaining metabolic health via regulation of body fat distribution. Through various tissue-specific knock out mouse models and mechanistic studies in primary cultured cells, they revealed Angpt2 produced from fat cells interacts with its receptor integrin α5β1 to drive fatty acid transporters and ensure normal distribution of circulating fat. "Intriguingly, Angpt2-integrin α5β1 signaling took just few minutes. This near-instant processing makes sense since this biological mechanism should cope with the surge of fat levels in the blood after a meal," explains Bae.
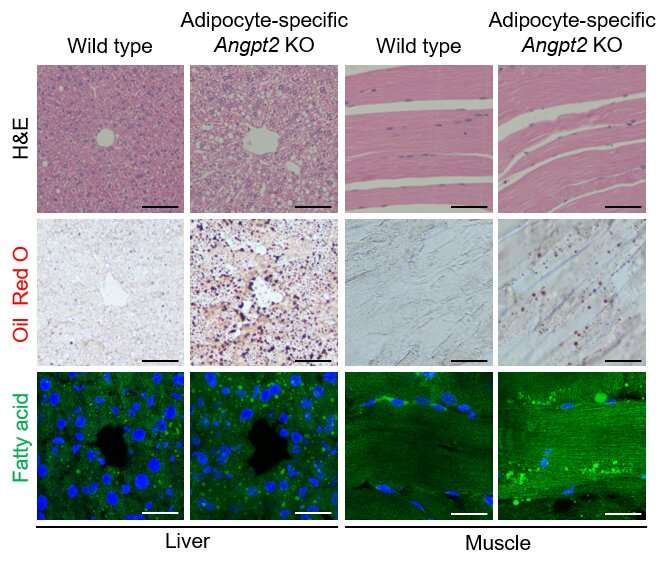
Figure 3. Depletion of Angiopoietin-2 from fat cells drives fat accumulation in other fat depots and abdominal organs. Representative images of morphology and fat accumulation into the liver (left) and muscle (right) in wild type and adipocyte-specific Angpt2 knockout mice. Fat accumulation in adipocyte-specific Angpt2 knockout mice could be observed in white spots (top), red oil staining (middle), and green fatty acid intensity (bottom). Credit: Institute for Basic Science
The inhibition of this process triggered fat accumulation in other fat depots and abdominal organs, leading to systemic glucose intolerance, which is reminiscent of the pattern in metabolically unhealthy obese patients.
Can Angpt2 treatment be a therapeutic strategy to normalize fat distribution and treat obesity-induced metabolic disorders? As shown in previous efforts, systemic modulation of Angpt2 through pharmacological blockade is limited as Angpt2 is individual, and it depends on heterogeneity of different fat depots. Alternatively, activating integrin receptors or fatty acid transporters may be a more feasible approach for its relatively restricted expression in fat cells under the skin. "It requires further investigation to test these possibilities genetically or pharmaceutically to open new therapeutic paths for transformation of metabolically unhealthy obesity to healthy obesity," adds Bae.
More information: Hosung Bae et al. Angiopoietin-2–integrin α5β1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistance, Nature Communications (2020). DOI: 10.1038/s41467-020-16795-4
Journal information: Nature Communications
Provided by Institute for Basic Science