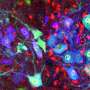
University of South Carolina scientists are exploring ways to make nerve regeneration happen faster and more successfully.
A new study published in Current Biology identifies the biological triggers that promote quicker nerve regeneration. From their previous studies, the researchers knew that damaged nerves regrow more quickly when "stress granules" in the site of the nerve injury are broken apart. Now they know what causes those stress granules to disassemble through a process called protein phosphorylation.
"The important thing is that we identified the protein that drives that process and showed how that's regulated," Jeff Twiss, a UofSC biology professor and co-author on the paper, said.
"It actually opens something new," Pabitra Sahoo, the paper's lead author, said. "In the future, it could help us design molecules that can promote phosphorylation."
Twiss said nerves typically regrow at 1 to 2 millimeters per day, meaning that an adult with nerve damage around their kneecap might require a year to recover as the nerve re-extends back to the foot. Given such a prolonged time to regenerate the nerve, atrophy makes a full recovery difficult.
"Finding ways to speed that up is critical to decreasing the amount of time that a person has loss of function, sensation and movement," said Twiss, the UofSC SmartState Chair in Childhood Neurotherapeutics. "But also, when you allow the nerve to find its way back to the target quicker, you can recover much more function."
Nerve cells contain the protein G3BP1 in clusters known as stress granules. When a nerve is severed, those granules begin to break apart through phosphorylation, a modification that makes G3BP1 become more negatively charged. This process releases mRNAs, important building blocks that the cell can use to build new proteins that extend the nerve. This phosphorylation makes the nerve grow faster, according to research that Sahoo and Twiss's team published in 2018.
The 2020 study took a step back to look for the processes that trigger the phosphorylation, in hopes that the entire process could be accelerated. The researchers determined that an enzyme known as Casein kinase 2-alpha (CK2α) is responsible for breaking up the G3BP1 granules through phosphorylation. When they increased CK2α levels, nerves grew faster, and the cell contained more phosphorylated G3BP1. When they decreased CK2α, the process slowed.
But where does the CK2α come from? The researchers placed a piece of nerve in a test tube, damaged it, and monitored the CK2α levels. Those levels increased, indicating that the damaged nerve synthesizes CK2α on its own at the injury site, rather than receiving it from its cell body. The process seems to be regulated by calcium ions.
These discoveries offer promising areas for further study. The UofSC researchers are already looking at methods for spurring the CK2α synthesis to speed up the nerve growth. Finding that key could lead to advances in medicine that result in faster healing after nerve injuries.
More information: Pabitra K. Sahoo et al. A Ca2+-Dependent Switch Activates Axonal Casein Kinase 2α Translation and Drives G3BP1 Granule Disassembly for Axon Regeneration. Current Biology. Published:October 15, 2020. DOI: 10.1016/j.cub.2020.09.043
Journal information: Current Biology
Provided by University of South Carolina