Credit: CC0 Public Domain
Trophoblast cells, which surround the developing blastocyst in early pregnancy, play an important role in implantation in the uterine wall. A new multidimensional model of trophoblast motility that utilizes a functionalized hydrogel is described in the peer-reviewed journal Tissue Engineering, Part A.
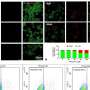
This valuable new tool, based on a methacrylamide-functionalized gelatin hydrogel, can be used for three-dimensional trophoblast spheroid motility assays. It can resolve quantifiable differences in outgrowth area and viability in the presence of a known invasion promoter and a known invasion inhibitor.
"Implantation involves a highly coordinated molecular dialogue between endometrial cells and trophoblast cells," state Brendan Harley and coauthors, University of Illinois at Urbana-Champaign. "Developing a deeper understanding of the biological mechanisms surrounding implantation may provide critical insights into pregnancy and pregnancy disorders."
"Dr. Harley and his colleagues at Illinois have provided a fundamental work to the growing field of pregnancy models, with a particular focus on the role of trophoblast migration. Here, the research team nicely showed that key factors—EGF and TGF-beta1—play a critical role in modulating trophoblast motility, and thus provide a pathway for better understanding these events during normal and complex pregnancies," says Tissue Engineering Co-Editor-in-Chief John P. Fisher, Ph.D., Fischell Family Distinguished Professor & Department Chair, and Director of the NIH Center for Engineering Complex Tissues at the University of Maryland.
More information: Samantha G. Zambuto et al, Tuning Trophoblast Motility in a Gelatin Hydrogel via Soluble Cues from the Maternal–Fetal Interface, Tissue Engineering Part A (2020). DOI: 10.1089/ten.tea.2020.0097
Provided by Mary Ann Liebert, Inc