Credit: Pixabay/CC0 Public Domain
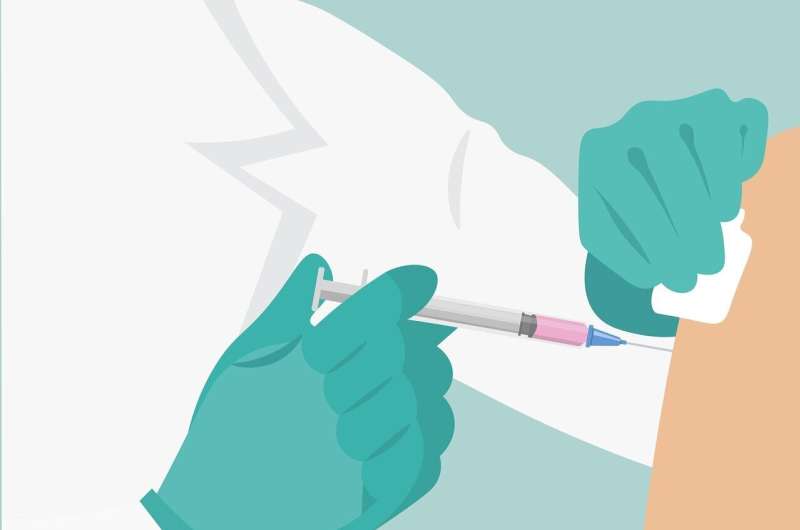
The European Union's medicines regulator said Friday it had started a "rolling review" of a COVID-19 vaccine developed by German firm CureVac, the first step towards a possible authorisation in the bloc.
"Preliminary results from laboratory studies and early clinical studies in adults... suggest the vaccine triggers the production of antibodies and immune cells that target" the coronavirus, the European Medicines Agency (EMA) said in a statement.
The rolling review of CureVac's shot will continue "until enough evidence is available for a formal marketing authorisation application", it added.
With delays to deliveries of three already-authorised vaccines, the EMA is under pressure from the EU's 27 member states to speed more into service.
CureVac's messenger RNA vaccine is currently undergoing large-scale Phase 3 trials, and would be produced by pharma giant Bayer if approved.
"If all goes well, EMA authorisation could come in April," Professor Peter Kremsner of the Tuebingen Institute of Tropical Medicine, who is supervising the trial, told German television.
The EMA itself said only that authorisation "should take less time than normal" thanks to the rolling review process, which is also being applied to the Novavax and Johnson and Johnson vaccines.
© 2021 AFP