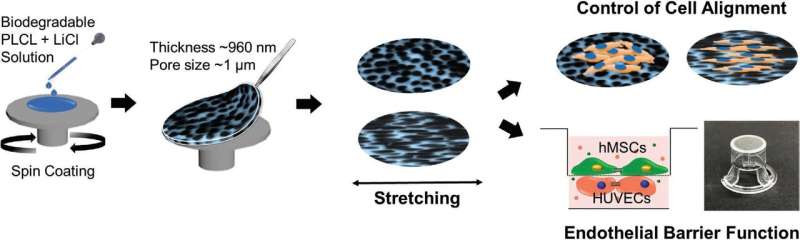
Overall schematic illustration of the fabrication of EPUM using PLCL using a VIPS process during spin-coating. The fabricated membrane was transferred to a 12-well insert for cell co-culture to mimic an endothelial barrier model. Credit: Korea Institute of Science and Technology(KIST)
A Korean research group has developed a technology that allows for the differentiation of stem cells into desired cell types, such as vascular mural cells or osteoblasts, without special pretreatment. This technology is expected to facilitate the production of artificial organs for preclinical studies or artificial tissues for transplants such as artificial skin and cardiac patches.
The Korea Institute of Science and Technology (KIST) announced that the research group led by Dr. Youngmee Jung of the Center for Biomaterials has developed a new cell co-culture platform based on porous, ultrathin membranes that can culture multiple types of cells simultaneously to form tissues similar to those native to the human body.
Cell co-culture, in which different types of cells are cultured together, is one of the methods used in making artificial organs that can be used as substitutes for animal-based preclinical studies, which are required for drug development. Because the human body consists of various types of cells, cell co-culture is crucial in simulating human tissues as closely as possible and is currently in use in most research fields that involve simulating biological tissues. However, simply mixing and culturing different types of cells together often causes fast-growing cells to overwhelm the others, leading to a lack of growth of the remaining cell types.
Among the cell culture platforms developed to resolve this issue, the platform using porous membranes had limitations in that the relative thickness of the membrane and the low density of the pores failed to induce active cell-cell interactions. Moreover, additional treatments were required to compensate for the differences between the environment of the platform and the in vivo environment, where cells actually grow.
To overcome such limitations, the researchers at KIST have developed a platform with membranes that are 10 times as thin as the existing ones and have a higher density of pores, enhancing cell-cell interactions. Using a soft and elastic polymeric material and enabling the elastic adjustments of the thin membrane, the new co-culture platform displays surface traits similar to those of the extracellular matrix, providing cells with an environment similar to that of the body. Furthermore, considering that tissues in blood vessels, muscles, heart, and other parts of the human body are often aligned in certain directions, the platform developed by the research group at KIST is suitable for culturing tissues as it can align cells without any further treatment, through pore alignment and nanoscale pattern formations using the elasticity of the membrane.
When vascular endothelial cells that form the inside of human blood vessels were co-cultured on this platform along with stem cells that can differentiate into vascular mural cells, the differentiation of stem cells into vascular mural cells was approximately 2.5 times greater than that observed on commercially available platforms, and vascular endothelial cells effectively formed cell-cell junctions, displaying a remarkable endothelial barrier function.
"This cell co-culture platform enables cell cultures that are simpler but more efficient than the currently available commercial platforms, and therefore displays high potential as an alternative tool for preclinical studies that can replace animal testing in pharmaceutical companies, hospitals, and other fields that require biological evaluations." said Dr. Youngmee Jung of KIST.
The results have been published in the latest issue of Advanced Functional Materials.
More information: Jin Yoo et al, Use of Elastic, Porous, and Ultrathin Co‐Culture Membranes to Control the Endothelial Barrier Function via Cell Alignment, Advanced Functional Materials (2020). DOI: 10.1002/adfm.202008172
Journal information: Advanced Functional Materials
Provided by National Research Council of Science & Technology