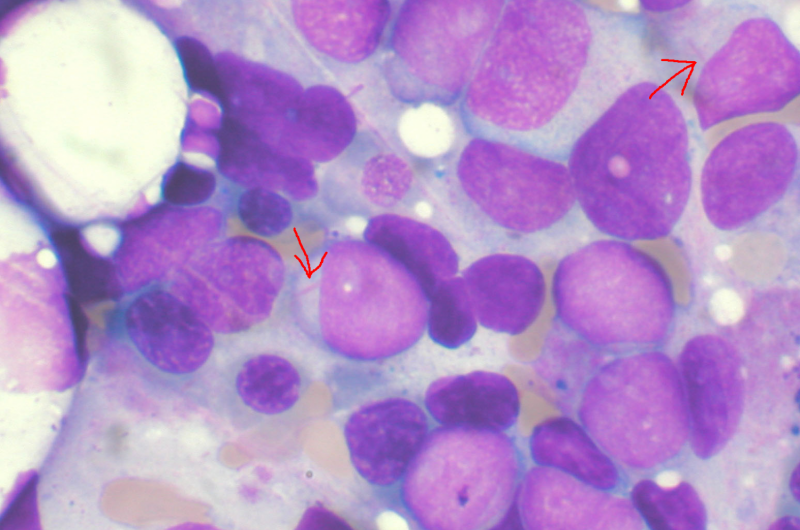
Bone marrow aspirate showing acute myeloid leukemia. Several blasts have Auer rods. Credit: Wikipedia
A team of Northwestern Medicine scientists led by Ali Shilatifard, Ph.D., the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics, has identified the molecular mechanisms of ASXL1 mutations involved in leukemia pathogenesis, according to a study published in Nature Cancer.
The findings also suggest that using small molecular inhibitors to block the BAP1 complex—a multi-protein complex that ASXL1 encodes a core nuclear protein for—may be a promising targeted therapy approach for leukemia.
"This is first inhibitor can benefit the treatment of leukemia patients with ASXL1 mutations," said Lu Wang, Ph.D., assistant professor of Biochemistry and Molecular Genetics and lead author of the study.
ASXL1 is one of the most frequently mutated genes in myeloid malignancies, including leukemia, and is associated with poor clinical outcomes. The gene encodes a 1,084-amino acid nuclear protein that is a core component of the BAP1 complex.
However, the molecular mechanisms that give rise to ASXL1 mutations that in turn alter BAP1 activity and drive the development of these diseases has remained understudied. Previous work regarding the role of ASXL1 mutations in regulating the BAP1 complex function has also remained unclear.
By developing new antibodies to recognize ASXL1 with cancer specific mutations, the investigators demonstrated that mutations in ASXL1 actually encode for gain-of-function proteins. Specifically, mutated ASXL1 proteins form a short peptide that binds to the BAP1 complex, enhancing and stabilizing the BAP1 complex, which activates gene expression and eventually promotes the development of leukemia.
Credit: Shilatifard Laboratory at NU
Based on this finding, the team collaborated with investigators led by Milan Mrksich, Ph.D., professor of Cell and Developmental Biology and vice president for Research at Northwestern, to perform a high throughput screening of more than 30,000 small molecule candidates and identified a first-in-class small molecule inhibitor that was able to inhibit BAP1 activity both in vitro and in vivo.
By applying the inhibitor to a xenograft model of ASXL1 mutations, they discovered that blocking BAP1 activity reduced the expression of genes upregulated due to ASXL1 mutations, ultimately suppressing tumor progression.
These findings reveal a novel potential therapeutic approach for treating leukemia and other solid tumors, said Shilatifard, adding that the team is currently developing an inhibitory compound to be approved for testing in clinical settings.
"If BAP1 activity causes cancer and ASXL1 mutations is the mechanism by which to get there, there's a large number of solid tumors and malignancies that are caused as a result of the ASXL1 mutation, and this inhibitor could be applicable to all of these cancers," said Shilatifard, who is also director of the Simpson Querrey Institute for Epigenetics.
More information: Lu Wang et al, Epigenetic targeted therapy of stabilized BAP1 in ASXL1 gain-of-function mutated leukemia, Nature Cancer (2021). DOI: 10.1038/s43018-021-00199-4
Journal information: Nature Cancer
Provided by Northwestern University