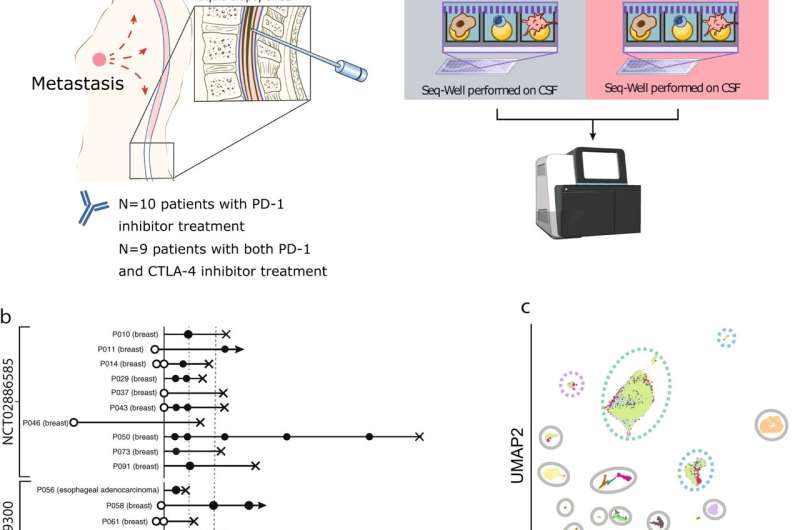
Fig. 1: Development of a pipeline for scRNA/cfDNA from longitudinally sampled CSF samples before and after ICI therapy. a Schematic representation of the longitudinal sampling performed on patients in this study. b Longitudinal sampling overview from patients in each study, including trial primary endpoint (dashed line), and date of patient mortality, when known. c UMAP of single-cell transcriptomes from all captured CSF cells in both trials, colored by patient, with cell type of origin indicated. Credit: DOI: 10.1038/s41467-021-25860-5
Two new studies indicate that immunotherapy may benefit people with leptomeningeal carcinomatosis (LMD), a rare but serious complication of cancer that has spread to the brain and/or spinal cord. The research, which was led by investigators at Massachusetts General Hospital (MGH), Dana-Farber Cancer Institute and the Broad Institute, is published in Nature Communications.
Although advances in cancer treatment have extended patient survival, some cancers come back, often in a different location in the body. This may in part help explain recent increases in the incidence of LMD—when tumor cells infiltrate the leptomeninges (layers of tissue that cover the brain and spinal cord) and cerebrospinal fluid. Approximately 5–8% of all patients with cancer develop LMD after first being diagnosed with breast cancer, lung cancer, melanoma or other malignancies. Current treatment options rarely benefit patients with LMD, and there is an urgent need for new therapies.
Immune checkpoint inhibitors are important medications that boost the immune system's response against various cancers, but their effects against LMD are unclear. To investigate, researchers conducted two phase II clinical trials. When they collected and analyzed immune cells and cancer cells from the cerebrospinal fluid of patients in the trials both before and after treatment with immune checkpoint inhibitors, the scientists found signs that the therapy was having an effect. For example, the number of certain cancer-killing immune cells and the expression of particular genes within cells were higher following treatment.
The second article in Nature Communications presents the results of one of the phase II studies, which included 18 patients with LMD who received combined ipilimumab and nivolumab (two types of immune checkpoint inhibitors) until the cancer progressed or the patient experienced unacceptable toxicity. The primary endpoint was overall survival at 3 months, and 8 of the 18 patients were alive at that time. (Historically, patients survive for a median of 3–7 weeks after being diagnosed with LMD.) One-third of patients experienced one or more serious adverse events. Two patients discontinued treatment due to unacceptable toxicity. The most frequent adverse events include fatigue, nausea, fever, anorexia and rash.
The authors noted that larger, multicenter clinical trials are needed to validate their results.
"In these two published studies, we demonstrated—in patients through a clinical trial and microscopically in the laboratory—that immune checkpoint blockade has promising activity for patients with LMD. More data is needed, but this is an exciting first step towards showing that immune checkpoint blockade may have a role in treating this devastating disease," says co-author Priscilla K. Brastianos, MD, who is the director of the Central Nervous System Metastasis Center at MGH and an associate professor of medicine at Harvard Medical School.
More information: Sanjay M. Prakadan et al, Genomic and transcriptomic correlates of immunotherapy response within the tumor microenvironment of leptomeningeal metastases, Nature Communications (2021). DOI: 10.1038/s41467-021-25860-5
Priscilla K. Brastianos et al, Phase II study of ipilimumab and nivolumab in leptomeningeal carcinomatosis, Nature Communications (2021). DOI: 10.1038/s41467-021-25859-y
Journal information: Nature Communications
Provided by Massachusetts General Hospital