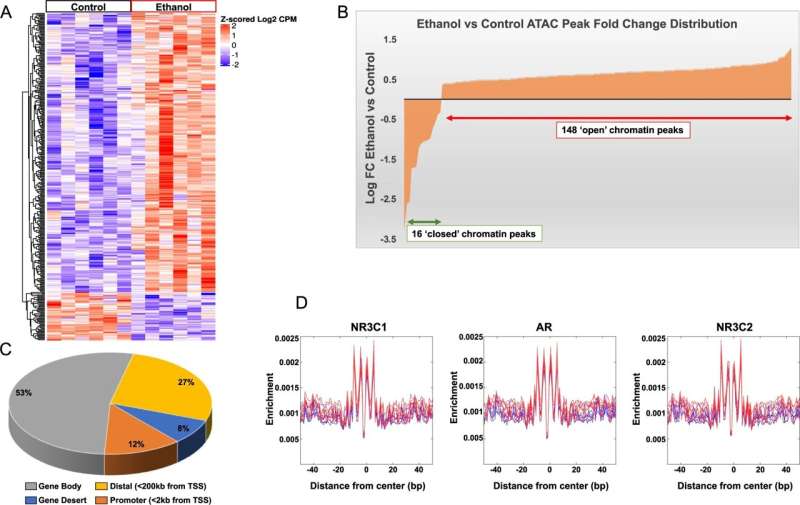
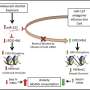
ATAC-seq data analysis in the amygdala of acute ethanol exposed rats. A Heatmap showing differential ATAC peaks (FDR < 0.2; 345 peaks) and chromatin accessibility in the amygdala after acute ethanol exposure. Red represents increases in peaks and blue represents decreases in peaks. Individual peaks are represented in rows and individual animals from control and ethanol groups (n = 6 animal per treatment) are shown in columns. B Distribution of ATAC peak fold changes indicating that out of the 164 peaks at FDR < 0.05, 148 peaks were associated with ‘open’ chromatin regions in the amygdala of acute ethanol-treated compared to 16 peaks in the control rats. C ATAC-seq data analysis in the amygdala of acute ethanol exposed rats showing distribution of ATAC peaks across genomic features. D Footprinting images for the top 3 (out of 41) motifs derived from FIMO analysis of 572 vertebrate motifs from the JASPAR database. The motifs satisfied the following criteria: q < 0.01 and >50% enrichment (log2 ratio >0.585). The blue lines represent controls and the red lines represent ethanol-treated samples. [(NR3C1- Glucocorticoid Receptor; Nuclear Receptor Subfamily 3 Group C Member 1), (AR- Androgen Receptor), NR3C2 (Mineralocorticoid Receptor; Nuclear Receptor Subfamily 3 Group C Member 2)]. Credit: Molecular Psychiatry (2022). DOI: 10.1038/s41380-022-01732-2
A new rodent study shows that even small quantities of alcohol can trigger epigenomic and transcriptomic changes in brain circuitry in an area that is crucial in the development of addiction.
What's more, the University of Illinois Chicago researchers who conducted the study say that the pathways involved in priming the brain for addiction are the same ones that are associated with the highs of drinking, like euphoria and anxiolysis, the clinical term for a level of sedation in which a person is relaxed but awake.
"This suggests that when the brain experiences the anti-anxiety effects of alcohol and the mood lift—the relaxation and the buzz—it is also being primed for alcohol use disorder," said the study's senior author Subhash Pandey, the Joseph A. Flaherty endowed professor of psychiatry and director of the Center for Alcohol Research in Epigenetics in the UIC College of Medicine.
While Pandey says the study does not suggest, for example, that one drink leads to addiction in humans, it does provide some clues as to why some people are more vulnerable to alcohol use disorder.
"We're seeing that dependent behaviors may not always be from long-term, high-quantity habits but a result of rapid epigenetic changes in the brain, which we show in this study may start happening even at low doses," said Pandey, who is also a senior research career scientist at the Jesse Brown Veterans Affairs Medical Center.
A paper published in Molecular Psychiatry details Pandey's experiments, which studied rats under control and alcohol exposure conditions.
In the experiments, rodents were exposed to low concentrations of alcohol and their behaviors were observed as they explored a maze. Then the researchers analyzed brain tissue samples, collected after euthanasia, with RNA sequencing and looked for gene expression patterns.
When they analyzed the samples, the researchers found that a gene called hypoxia inducible factor 3 alpha subunit—Hif3a, for short—was associated with changes in the brain after alcohol exposure and behaviors, like how long rats stayed in areas of the maze with enclosed (high anxiety) or open arms (low anxiety).
Alcohol increased Hif3a expression, even after low doses of exposure, and reduced anxiety. And, while many effects of alcohol are different among males and females, there was no difference between the two in this study.
"We saw that low doses, what we consider 'social drinking,' changes the gene expression in the amygdala, a brain region that regulates anxiety. In other words, it creates an epigenetic pathway for addiction," Pandey said.
Pandey and his colleagues also set up additional experiments in which they blocked the gene in the amygdala of rats with or without alcohol exposure to validate its role mediating anxiety. When Hif3a was blocked, anxiety was increased in control rats, mimicking withdrawal from chronic alcohol exposure. On the other hand, this also prevented the anti-anxiety effects of alcohol.
The researchers showed why, too. Hif3a's chromatin—bundles of DNA and RNA—is loosely bundled, meaning the genes are easily accessible for transcription changes.
One thing the study does not suggest, however, is what level of alcohol exposure was safe for rodents. Instead, Pandey said, it's important to know that low doses created a priming for addiction. For people, he thinks the takeaway is simple—don't assume social drinking or even "pandemic drinking" is without risk.
"Alcohol use disorder is complex and challenging to overcome. The information we learned from this study helps us to understand better what is happening in the brain and, one day, may be leveraged to develop better treatments and pharmaceuticals," Pandey said.
More information: Harish R. Krishnan et al, Unraveling the epigenomic and transcriptomic interplay during alcohol-induced anxiolysis, Molecular Psychiatry (2022). DOI: 10.1038/s41380-022-01732-2
Journal information: Molecular Psychiatry
Provided by University of Illinois at Chicago