Credit: Pixabay/CC0 Public Domain
The heart disease arrhythmogenic cardiomyopathy can lead to sudden death, particularly affecting young athletes. Researchers at the University of Basel have now genetically modified mice, which develop a similar disease to that found in humans. This allowed the team to identify previously unknown mechanisms and potential therapeutic targets.
In August 2007, a soccer match took place that fans of the club Sevilla FC will not forget: the 22-year-old Antonio Puerta suffered a cardiac arrest and collapsed on the field, passing away in hospital a few days later. It was later discovered that the player was affected by a condition named arrhythmogenic cardiomyopathy.
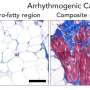
This inherited disease is estimated to occur in one of 5,000 people, with men being more commonly affected than women. "Arrhythmogenic cardiomyopathy leads to arrhythmia with a loss of cardiac muscle cells, deposits of connective tissue and fat within the cardiac muscle. This can cause sudden cardiac death, often during exercise," says Volker Spindler, anatomist and head of the Cell Adhesion group at the University of Basel's Department of Biomedicine.
Today, a range of gene mutations are known to trigger the condition. However, even with an early diagnosis there is no cure, only options for the management of symptoms are available. "Patients are advised to avoid any competitive or endurance sports and have to take medications such as beta blockers. Where appropriate, a catheter ablation may be performed or an implantable defibrillator may be used," says the cardiologist Gabriela Kuster, who heads the Myocardial Research group at the Department of Biomedicine. Sometimes the only option is a heart transplant.
Cardiac muscle cells lose their stickiness
The starting point for the project was the notion that many of the mutations affect structures known as the desmosomes. These are protein clusters on the surface of cardiac muscle cells that ensure a tight connection between the cells. "You can imagine these clusters to act like a piece of Velcro," says the physician Dr. Camilla Schinner, first author of the study just published in the journal Circulation. This led to the theory that the mutations reduce adhesion between the cells, thus weakening the cardiac muscle.
To test this hypothesis, Spindler's team introduced a mutation similar to that found in patients into the genome of mice. The cardiac function of these animals was then examined by Kuster's group. The result: the genetically modified animals showed a heart disease with arrhythmia that resembled arrhythmogenic cardiomyopathy in humans. In addition, microscopic and biochemical analysis indeed showed reduced adhesion between the cardiac muscle cells. The researchers also observed the scarring of the cardiac muscle typical for this disease.
Preventing cardiac tissue damage
Their next step was to investigate how diseased cardiac muscle differed from healthy conditions at the molecular level. Mice with the mutation showed an increased amount of a particular protein at the Velcro-like structures of the heart muscle cells. This leads, via a series of events, to connective tissue deposition and scarring of the heart. The addition of a substance which blocks this cascade prevented disease progression—which is why Spindler here sees a potential new treatment approach.
"Nevertheless, there is still a long way to go until an application in humans may be considered," he points out. "But we now have better options to study the disease in more detail to improve our understanding of the underlying mechanisms."
More information: Camilla Schinner et al, Defective Desmosomal Adhesion Causes Arrhythmogenic Cardiomyopathy by Involving an Integrin-αVβ6/TGF-β Signaling Cascade, Circulation (2022). DOI: 10.1161/CIRCULATIONAHA.121.057329
Journal information: Circulation
Provided by University of Basel