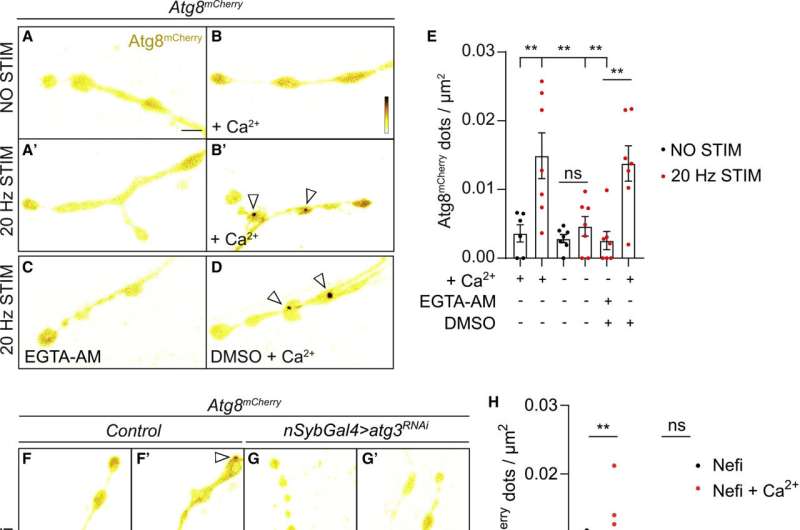
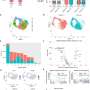
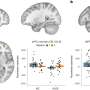
(A–E) Live imaging of non-stimulated and stimulated (30 min, 20 Hz) Drosophila larval NMJ boutons expressing Atg8mCherry at endogenous levels in the absence of Ca2+ (A and A′), presence of Ca2+ (B and B′), presence of EGTA-AM (no Ca2+ in the buffer) (C), and presence of DMSO plus Ca2+ (D). Fluorescence intensities shown using scale (0–1292 gray value) indicated in (B). Quantification of the number of Atg8mCherry dots (arrowheads) (E). Error bars: mean ± SEM; scale bar: 5 μm. Statistical significance: one-way ANOVA with Tukey’s multiple comparison test: ∗∗ p < 0.01, ns, not significant, n ≥ 6 larvae (24 NMJs) per genotype. (F–H) Live imaging of genomically expressed Atg8mCherry in NMJ boutons of w controls (F and F′) and of animals expressing RNAi against atg3 (under the control of the pan-neuronal driver nSyb-Gal4) (G and G′). Non-stimulated animals were incubated for 30 min in HL3 containing nefiracetam (Nefi) (10 μM) and post-synaptic glutamate receptor blocker 1-naphthylacetyl spermine trihydrochloride (NAS) (100 μM) to block muscle contractions (F and G). Stimulated animals were incubated for 30 min in HL3 with Nefi (10 μM), NAS (100 μM), and CaCl2 (1 mM) (F′ and G′). Quantification of the number of Atg8mCherry dots (arrowheads) (H). Error bars: mean ± SEM; scale bar: 5 μm. Statistical significance: two-way ANOVA with Šidàk multiple comparison test: ∗∗ p < 0.01, ns, not significant, n ≥ 9 larvae (36 NMJs) per genotype. (I–K) Live imaging of genomically expressed Atg8mCherry in NMJ boutons of w control animals (I and I′) and of endoA−/− null mutant animals expressing phospho-dead endoAS75A (genomic construct) (J and J′). Quantification of the number of Atg8mCherry dots (arrowheads) (K). Error bars: mean ± SEM; scale bar: 5 μm. Statistical significance: two-way ANOVA with Šidàk multiple comparison test: ∗∗ p < 0.01, ns, not significant, n ≥ 11 larvae (44 NMJs) per genotype. Credit: Neuron (2023). DOI: 10.1016/j.neuron.2023.02.001
Researchers from the University of Queensland have identified that a gene associated with an increased risk of Parkinson's disease also contributes to a build-up of cell debris in the brain.
Dr. Adekunle Bademosi from The Queensland Brain Institute said the discovery could change the focus of Parkinson's disease treatment.
"Our team has found that a Parkinson's disease-linked mutation in a gene called Endophilin A1 blocks the process by which the body and the brain recycle cell waste," Dr. Bademosi said. The study was published in Neuron.
Without the process, called autophagy, toxic debris builds up and neurons die—known hallmarks of Parkinson's disease.
"We knew we could induce autophagy in cells by starving them of amino acids and the subsequent breakdown of debris tells a protein called EndoA to approach the cell membrane and begin the recycling process," Dr. Bademosi said.
"Now we've also seen that regular signals between neurons in the brain starts EndoA-induced autophagy when the electric impulses trigger the release of proteins or neurotransmitters at synapses.
"Unfortunately, when the Endophilin A1 gene is affected in Parkinson's, the protein EndoA becomes insensitive to this trigger at the synapse and the debris that should be thrown out for recycling builds up instead."
In vivo single particle tracking of EndoA. Credit: Neuron (2023). DOI: 10.1016/j.neuron.2023.02.001
Current Parkinson's treatments tend to focus on clearing out the build-ups and replacing what is lost when too many neurons die.
"It may be time to shift the treatment focus to autophagy as the mechanism underlying these disease hallmarks," Dr. Bademosi said. "Exploring the use of compounds that induce or inhibit autophagy could pave the way for new, more effective Parkinson's drugs."
More information: Adekunle T. Bademosi et al, EndophilinA-dependent coupling between activity-induced calcium influx and synaptic autophagy is disrupted by a Parkinson-risk mutation, Neuron (2023). DOI: 10.1016/j.neuron.2023.02.001
Journal information: Neuron
Provided by University of Queensland