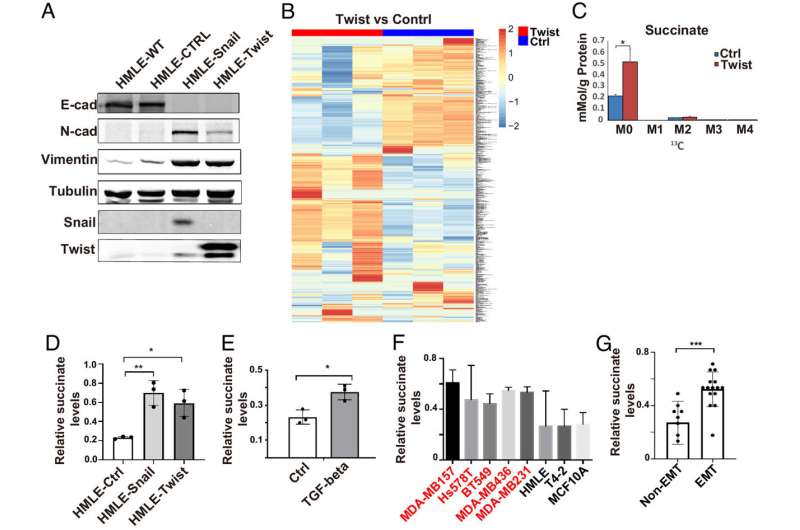
Succinate levels are elevated during EMT. (A) Expression of EMT markers was assessed by western blot in control, Snail1- and Twist1-expressing HMLE cells. (B) SIRM analysis showed the differential accumulation of metabolites in control and Twist1-expressing HMLE cells. (C) Quantification of succinate levels in control and Twist1-expressing HMLE cells with SIRM. M0 is unlabeled succinate, M2 is succinate with 2 13C atoms; results are presented as mean ± SEM; n = 3; *P < 0.05, independent t test. (D and E) Cytoplasmic succinate levels were quantified in control, Snail- or Twist1-expressing HMLE cells, and TGF-β-treated HMLE cells. Results are presented as mean ± SEM; n = 3; *P < 0.05, **P < 0.01, one-way ANOVA. (F and G) Quantification of cytoplasmic succinate levels in a panel of mammary epithelial cell lines with or without mesenchymal phenotypes; the cells lines with mesenchymal phenotypes are labeled in red. Results are presented as mean ± SEM; n = 3; ***P < 0.001, independent t test. Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2214942120
A new University of Kentucky Markey Cancer Center study reveals more about changes that happen to cancer cells when they metastasize and identifies a promising target for the treatment of metastatic breast cancer.
Metastasis is when cancer cells spread from the primary tumor to surrounding tissues and distant organs in the body and is the primary cause for breast cancer mortality. Cancer cells' plasticity, or their ability to change and adapt, is critical for progression to metastatic cancer.
The research, published in PNAS on May 8, shows a metabolite called succinate plays a role in enhancing cancer cell plasticity and identifies an enzyme called PLOD2 as a regulator of succinate during breast cancer progression.
"The results reveal a previously unidentified function of succinate and its role in breast cancer metastasis, filling a critical gap in our knowledge regarding how changes in metabolites promote cancer cell plasticity," said Ren Xu, Ph.D., the study's principal investigator and a professor in the UK College of Medicine's Department of Pharmacology and Nutritional Sciences.
In collaboration with Andrew N. Lane, Ph.D., professor in the College of Medicine's Department of Toxicology and Cancer Biology and co-director of the Center for Environmental and Systems Biochemistry, Xu's team studied metabolic reprogramming in mammary epithelial cells during epithelial mesenchymal transition (EMT), a biologic process that mobilizes cancer cells.
The results also suggest targeting PLOD2 could be a promising strategy to suppress breast cancer metastasis and drug resistance.
PLOD2 expression during EMT elevated succinate levels in breast cancer cells, while PLOD2 inhibition reduced succinate levels and inhibited cancer cell plasticity.
"As PLOD2 is a druggable target, these findings could pave the way for the development of new therapies to stop cancer progression," said Xu.
More information: Yuxin Tong et al, The PLOD2/succinate axis regulates the epithelial–mesenchymal plasticity and cancer cell stemness, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2214942120
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Kentucky