Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rebound occurs at a similar rate for those receiving and not receiving oral antiviral treatment and for those receiving nirmatrelvir/ritonavir or placebo, according to research published in the Dec. 22 issue of the U.S. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report.
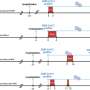
Dallas J. Smith, Pharm.D., from the CDC in Atlanta, and colleagues reviewed SARS-CoV-2 rebound studies published during Feb. 1, 2020, to Nov. 29, 2023, to enhance current understanding of rebound. Seven studies met the inclusion criteria: one randomized trial and six observational studies, which compared rebound for those receiving and not receiving antiviral treatment. The researchers observed no significant differences in rebound rates for those receiving versus not receiving treatment in four studies, including the randomized trial. Outpatients who experienced rebound had no hospitalizations or deaths reported.
Patrick R. Harrington, Ph.D., from the U.S. Food and Drug Administration in Silver Spring, Maryland, and colleagues examined viral RNA shedding from two phase 2/3 placebo-controlled, randomized trials of nirmatrelvir/ritonavir to examine the role of treatment in COVID-19 rebound. The researchers found that patients receiving nirmatrelvir/ritonavir or placebo had similar rates of SARS-CoV-2 RNA shedding based on nasopharyngeal viral RNA levels from day 5 (end of treatment) to day 10 or day 14. Viral RNA rebound occurred in 6.4 to 8.4 percent of nirmatrelvir/ritonavir recipients and 5.9 to 6.5 percent of placebo recipients among those with a virologic response through day 5.
"These findings support FDA's determination of safety and efficacy of nirmatrelvir/ritonavir in eligible patients at high risk for severe COVID-19," Harrington and colleagues write.
More information: Dallas J. Smith et al, SARS-CoV-2 Rebound With and Without Use of COVID-19 Oral Antivirals, MMWR. Morbidity and Mortality Weekly Report (2023). DOI: 10.15585/mmwr.mm7251a1
Patrick R. Harrington et al, Evaluation of SARS-CoV-2 RNA Rebound After Nirmatrelvir/Ritonavir Treatment in Randomized, Double-Blind, Placebo-Controlled Trials—United States and International Sites, 2021–2022, MMWR. Morbidity and Mortality Weekly Report (2023). DOI: 10.15585/mmwr.mm7251a2
Journal information: Morbidity and Mortality Weekly Report
Copyright © 2023 HealthDay. All rights reserved.