This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Viral rebound and safety of nirmatrelvir/ritonavir for lung-transplant recipients infected with SARS-CoV-2
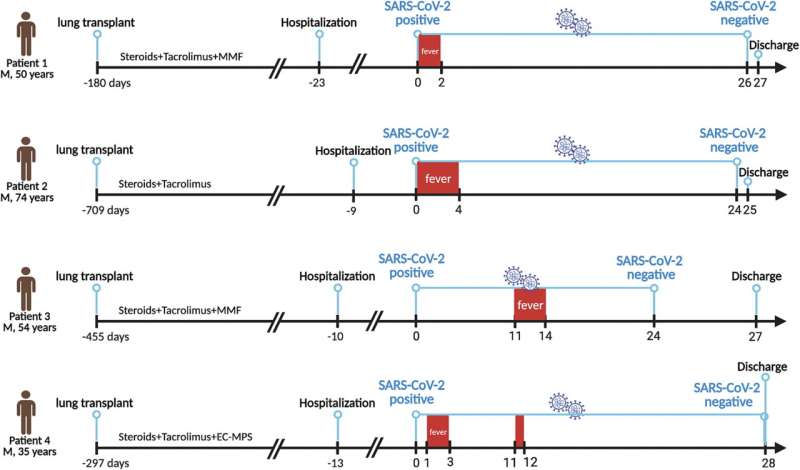
Data on the viral rebound and safety of nirmatrelvir/ritonavir in lung transplant (LTx) recipients are limited. A study published in Biosafety and Health prospectively followed four LTx recipients. Clinical characteristics, viral RNA dynamic in throat swabs, and tacrolimus blood concentration were monitored regularly.
All four LTx recipients, aged 35–74 years, were not vaccinated against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). They acquired coronavirus disease 2019 (COVID-19) after more than one week of admission during the era of omicron.
All cases received nirmatrelvir/ritonavir (NM/r) within two days of infection, and the relative viral RNA copies dropped quickly. Viral load rebound was observed in all four cases after discontinuation of the first five days of NM/r treatment. Three of them received another 5-days antiviral therapy with NM/r. The duration of positive viral PCR testing was 25–28 days.
None of them progressed into severe or critical COVID-19. Tacrolimus was stopped 12 h before NM/r and held during the 5-day course of antiviral therapy. Blood concentration of tacrolimus were maintained at a baseline level during these five days. Tacrolimus was re‐initiated at its baseline daily dose 3–4 days after NM/r therapy. However, during the second round of antiviral therapy with NM/r, the concentration of tacrolimus fluctuated wildly.
In conclusion, the 5-day course of NM/r treatment was not sufficient for LTx recipients and the viral rebound was common. More data are needed to clarify whether LTx recipients with SARS-CoV-2 viral rebound could benefit from additional treatment with NM/r.
More information: Hui Li et al, Viral rebound and safety of nirmatrelvir/ritonavir for lung-transplant recipients infected with SARS-CoV-2, Biosafety and Health (2023). DOI: 10.1016/j.bsheal.2023.08.004