This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Virologic rebound observed in 20% of patients treated with nirmatrelvir-ritonavir

An observational study of patients being treated for acute COVID-19 in a multicenter health care system observed virologic rebound in about 20% of patients treated with nirmatrelvir-ritonavir (N-R) versus about 2% of those who did not receive treatment. The findings are published in Annals of Internal Medicine.
N-R is an oral antiviral widely used in the United Sates to reduce the incidence of hospitalization and death among individuals with mild to moderate COVID-19. Soon after its adoption into clinical care, a clinical and virologic rebound phenomenon was reported, but data are conflicting about how common it is.
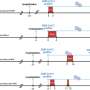
Researchers from Massachusetts General Hospital and Brigham and Women's Hospital studied 127 patients with acute COVID-19 to compare the frequency of virologic rebound in patients with (n=72) and without (n=55) N-R treatment. The study outcome was viral rebound within 3 weeks of an initial positive test, defined as either a positive SARS-CoV-2 viral culture following a prior negative culture, or sustained elevated viral load after a prior decline.
With frequent monitoring by both PCR and viral culture during the acute stages of COVID-19, the data showed that virologic rebound with replication-competent prolonged viral shedding occurred in approximately 1 in 5 individuals taking N-R. Only one untreated patient experienced virologic rebound. People who rebounded shedded live virus for a median of 14 days versus less than five days in those who did not.
A regression model showed a trend towards higher rates of virologic rebound with earlier N-R initiation after the date of diagnosis and with earlier N-R initiation after the onset of symptoms. The researchers noted that compared with untreated individuals, those taking N-R were older, had received more COVID-19 vaccinations, and were more often immunosuppressed.
According to the study authors, these findings should be factored into consideration when weighing the benefits and risks of N-R treatment in patients at low risk for severe disease. Still, for patients at moderate to high-risk for severe COVID-19, the clinical benefits associated with N-R use are well established.
More information: SARS-CoV-2 Virologic Rebound With Nirmatrelvir–Ritonavir Therapy, Annals of Internal Medicine (2023). www.acpjournals.org/doi/10.7326/M23-1756
Editorial: Annals of Internal Medicine (2023). https://www.acpjournals.org/doi/10.7326/M23-2887