This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
written by researcher(s)
proofread
Nanoscopic motor proteins in the brain build the physical structures of memory, study finds
The puzzle of memory has intrigued philosophers and intellects for a very long time. Plato and Aristotle believed that memory was found only in the realm of the soul and the mind, but there was nothing corporeal or physical about it. Memory is closely tied to our sense of self and subjective experiences, but there are physical processes that are associated with remembering.
Modern analogy likes to compare computer memory to that of the brain, where the activity of brain cells called neurons are compared to the binary codes of the magnetic field patterns stored in a hard drive. However, computer devices don't change as a result of performing their jobs, unlike neurons.
Storing and processing memories uses nanoscopic motor proteins called kinesin that move materials around within neurons to construct the structural code of memory. These nanoscopic workers "walk" using alternating steps on long molecular tracks to deliver materials.
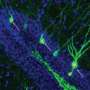
For 20 years, neuroscientists—including myself—have used cutting-edge microscopy technology in living animals to observe microscopic structures called dendritic spines constantly budding out, morphing and regressing on the dendrites of neurons.
Dendritic spines are where neurons form contacts with other neurons and create electrical circuits throughout the brain. Dendritic spine plasticity, as this change of dendrite shape is referred to, is more than the random movement of neuronal structures in the brain.
Budding spines to store new memories
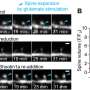
In our recently published study in Cell Reports, the extent of dendritic spine plasticity is found to be highly correlated with memory performance of animals in our lab. We taught mice to fear a harmless tone by electrocuting them each time the tone was played; then, we taught the mice to overcome the same fear by presenting the same tone repeatedly in a harmless situation.
After two days, the degree of fear, as indicated by the length of time the mice were immobile for, represents memory performance. The greater the number of dendritic spines that budded on neurons, the shorter the amount of time they remained frozen.
Similarly, scientists discovered that motor memory—as indicated by how long mice can run on a rotating rod after training—is also correlated with the number of budding dendritic spines on neurons.
When these newly formed dendritic spines are scratched using a sophisticated technology called optogenetics, mice lose their motor memory and perform as if they had not trained at all.
This evidence strongly impacts how we understand the way memory is stored. Beyond the all-or-nothing activity of a whole neuron, the structural traces of memory are formed by the patterns of microscopic structures called dendritic spines on neurons.
Delivering molecular cargoes to spines
This discovery raised another challenge: how do neurons know where specifically on their branches to "build" these memory codes? These locations must be specific as they correspond to contact points with different neurons in forming neural circuits relevant to different experiences.
Since most cellular materials are synthesized in the cell body, there must be a transporter to deliver materials within neurons to achieve the accurate construction of memory codes.
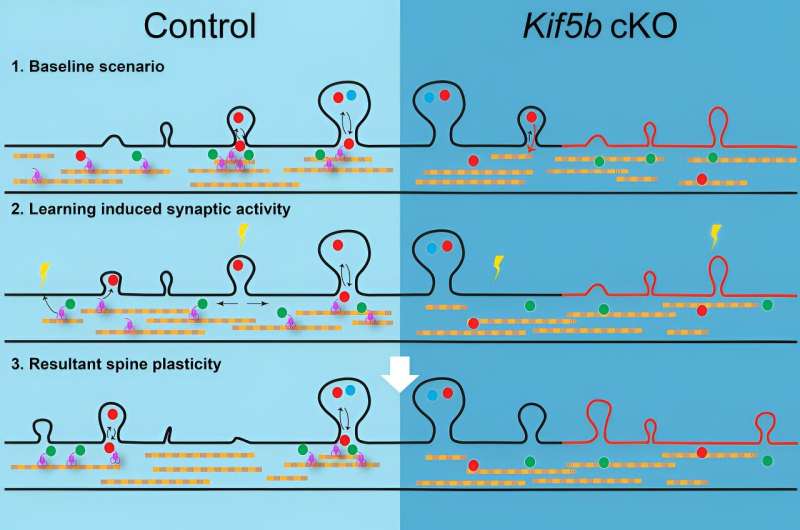
In our study, we speculated that kinesin was used to deliver molecular materials for "building" dendritic spines. To prove this, we tagged molecular cargoes known to be carried by kinesin with fluorescent markers, so that we could follow the movement of kinesin under the microscope. Using this cutting-edge microscopy technology, we could track the movement of kinesin in the brain before and after creating and removing fear in the mice.
We also genetically removed kinesins from another group of mice to understand if the function of kinesin was indeed necessary for forming dendritic spine memory code. We found that in normal mice with kinesin, it took several hours as opposed to minutes for kinesin to move to a specific location on dendrites, where dendritic spines can bud out. If kinesin was removed from the brain, the tagged molecular cargoes showed reduced movement and as a result, the number of dendritic spines formed was completely altered and the stability of the ones formed was significantly hampered.
Without kinesin, the mice could not learn or form memory properly in our study.
Understanding memory
This is the first time that the process of structural memory code formation has been visualized, identifying kinesin as the transport for building dendritic spines following a learning experience to construct the structural code of memory in the living brain. This structural memory code can provide an even more complex dimension than binary encoding of information.
Further understanding and potential mapping of these dendritic spine structural codes on a large scale in the brain could open up new ways to manipulate memory functions in medical conditions.
More information: Albert Hiu Ka Fok et al, KIF5B plays important roles in dendritic spine plasticity and dendritic localization of PSD95 and FMRP in the mouse cortex in vivo, Cell Reports (2024). DOI: 10.1016/j.celrep.2024.113906
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()