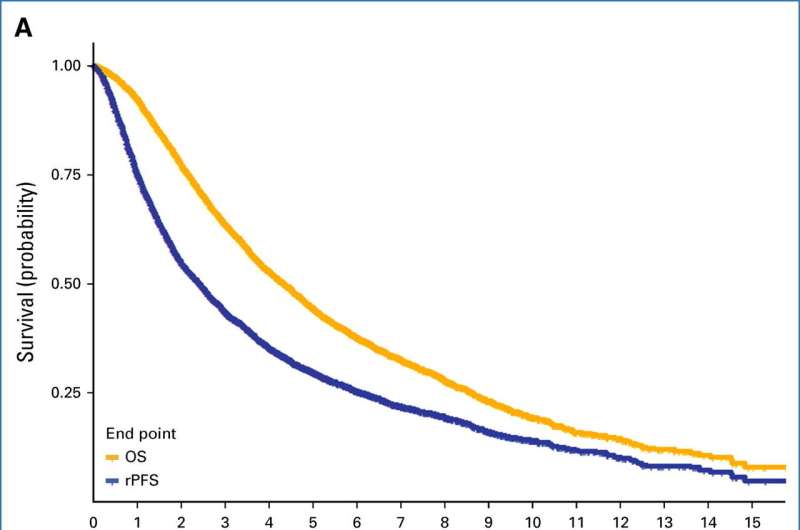
Kaplan-Meier curves for (A) OS and rPFS and (B) OS and cPFS. cPFS=clinical progression-free survival; OS=overall survival; rPFS=radiographic progression-free survival. Credit: Journal of Clinical Oncology (2024). DOI: 10.1200/JCO.23.01535
A lack of cancer progression could be used as a substitute for overall survival in metastatic hormone-sensitive prostate cancer clinical trials, according to a new meta-analysis published in the Journal of Clinical Oncology, an approach that could accelerate research and allow new treatments to reach patients faster.
Out of every 100 American men, roughly 13 will get prostate cancer during their lifetime, according to the Centers for Disease Control and Prevention, and about two to three of those will die from the cancer.
While there is no cure, prostate cancer can be slowed by lifelong hormone therapy, which works by suppressing the production of testosterone.
Because of recent advances in treating prostate cancer, patients survive longer than ever before, said Maha Hussain, MD, the Genevieve E. Teuton Professor of Medicine in the Division of Hematology and Oncology and a co-author of the new study. This means that clinical trials measuring how long patients live after an experimental treatment can take decades, slowing overall research progress.
"In the older days, when we didn't have the strong treatments we have now—men with metastatic hormone-sensitive prostate cancer survived on average for two and a half to three years," said Hussain, who is also deputy director of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
"Now, on average they survive about six years and some may survive for a decade with treatment. So, to do a clinical trial and wait for the overall survival on average, you're really waiting a decade plus to get the outcomes."
In the current study, Hussain and her collaborators sought to understand if a lack of cancer progression as monitored by radiography could substitute overall survival as a metric for metastatic prostate cancer clinical trials.
Investigators analyzed data from more than 6,300 patients involved in nine previous prostate cancer hormonal therapy clinical trials in the last three decades and found that radiographic progression-free survival and clinical progression-free survival were comparable stand-ins for overall survival, according to the study.
"Essentially, the bottom line is: both radiographic progression-free survival and progression-free survival can potentially be used as an endpoint because they are strong surrogates for overall survival," Hussain said.
Moving forward, Hussain said she hopes the new criteria will accelerate clinical trials conducted for metastatic prostate cancer and allow new treatments to reach patients who need them faster.
"We've come a long way with prostate cancer," Hussain said. "I'm hopeful that with new, powerful agents, we will have a cure at some point. At least for now, patients are living much longer."
More information: Susan Halabi et al, Radiographic Progression-Free Survival and Clinical Progression-Free Survival as Potential Surrogates for Overall Survival in Men With Metastatic Hormone-Sensitive Prostate Cancer, Journal of Clinical Oncology (2024). DOI: 10.1200/JCO.23.01535
Journal information: Journal of Clinical Oncology
Provided by Northwestern University