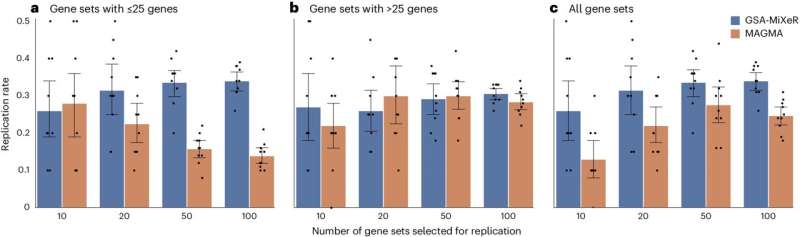
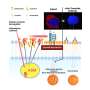
Replication analysis for schizophrenia. Replication rate is computed for GSA-MiXeR and MAGMA analyses using twofold cross-validation, after applying both methods to two independent and equally sized GWAS subsamples. After ranking gene sets according to fold enrichment of heritability (GSA-MiXeR) or enrichment P value (MAGMA) obtained in the discovery sample, the replication rate was then defined as the fraction of gene sets that remain within top N gene sets in the replication half of the dataset. Credit: Nature Genetics (2024). DOI: 10.1038/s41588-024-01771-1
Researchers from the University of Oslo have developed an innovative method to improve our understanding of heritable human traits and diseases. The analytical tool, called GSA-MiXeR, is designed to make sense of genetic data by focusing on the role of individual genes, and how groups of genes contribute to the risk of developing a disease. With it, researchers now have a powerful new way to translate genetic research into practical insights that could lead to better treatments for a range of complex diseases.
The findings are published in the journal Nature Genetics.
More than 970 million people worldwide are living with a mental illness, according to WHO, and the global burden of these diseases is considerable. While researchers have been successful in identifying genetic factors associated with conditions such as schizophrenia through genome-wide association studies (GWAS), figuring out what these discoveries mean for our health is still a big challenge.
"GWAS, which are often produced by large international consortia, analyze the genomes of many individuals to find genetic variations associated with specific diseases. Our tool, called GSA-MiXeR, is designed to analyze the genetic data collected from these large-scale studies, aiming to identify how groups of genes contribute to the risk of developing a disease," says Oleksandr Frei, a researcher at the Center for Precision Psychiatry at the University of Oslo.
Complex polygenic traits, which are influenced by many genetic factors, have been particularly difficult to interpret. "GSA-MiXeR addresses this by providing a clearer picture of how different genes work together," he explains.
When applied to a variety of complex traits and diseases, including schizophrenia, GSA-MiXeR has been able to highlight specific gene groups that are more closely related to the disease than traditional methods have been able to.
One example is how GSA-MiXeR identified that genes involved in controlling calcium channels in our cells and those involved in dopamine signaling, play a significant role in schizophrenia. "These findings are not just important for understanding the disease—they also may point to potential targets for developing new treatments," Frei says.
Better understanding of complex traits and disorders can lead to precision medicine, where treatments are tailored to the genetic makeup of individual patients. "This approach can improve the effectiveness of treatments and reduce side effects. By translating genetic research into practical insights, GSA-MiXeR can contribute to more personalized and effective health care, ultimately leading to better health outcomes for patients," Frei says.
With GSA-MiXeR, scientists now have a powerful new way to translate genetic research into practical insights that could lead to better treatments for a range of complex diseases.
More information: Oleksandr Frei et al, Improved functional mapping of complex trait heritability with GSA-MiXeR implicates biologically specific gene sets, Nature Genetics (2024). DOI: 10.1038/s41588-024-01771-1
Journal information: Nature Genetics
Provided by University of Oslo