This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Transposons could be new targets for aging research and treatment
A new USC Leonard Davis School-led study highlights how transposons—commonly called "jumping genes" because of their ability to move to different parts of the genome—are associated with age-related disease and decline, as well as how additional genes governing transposon expression may one day be therapeutic targets for aging.
The study, "An eQTL-based approach reveals candidate regulators of LINE-1 RNA levels in lymphoblastoid cells," was published in PLOS Genetics on June 7, 2024.
Transposons make up approximately 45% of human DNA, and their activity is largely repressed in younger, healthy cells. However, with age, these genes are expressed more and become more mobile, correlating with various age-related declines in function, said Bérénice Benayoun, the study's corresponding author.
"The question is, 'Is increased transposon activity just a byproduct of aging, or could it actually drive aspects of aging?'" she said.
Existing data yields new surprises
Benayoun, associate professor of gerontology, biological sciences, biochemistry and molecular medicine, said the COVID-19 pandemic was part of the inspiration for the study.
While lockdowns meant time in the laboratory was harder to come by, Benayoun and her team turned to a "treasure trove" of existing human genome data (which genes are present in DNA) and transcriptome data (which genes are active and transcribed into RNA for producing proteins) that could be studied remotely with computer analysis.
The team focused on long interspersed element 1 (LINE-1), a family of transposons that collectively make up about 17% of the human genome. Previous studies have shown that, like other transposons, LINE-1 also appears to be expressed more with age and in aging-related disease.
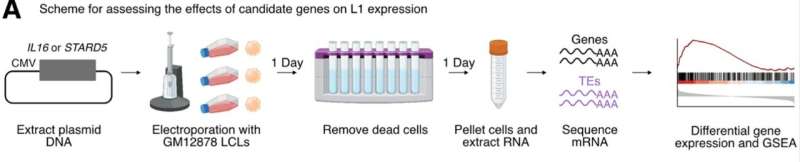
Juan Bravo, postdoctoral fellow in the Benayoun lab and the study's first author, designed an analysis using DNA and RNA sequencing data from human lymphoblasts, a type of immune cell. The expression quantitative trait loci (eQTL) analysis he created pinpointed several genes that affect how much LINE-1 is expressed.
"Transposons have historically been ignored for several reasons, one of which is the additional and technical complexity of quantifying transposon RNA levels," Bravo said.
"Nevertheless, by including both protein-coding genes and transposons in our analysis, we were able to add a layer to our analysis where we could identify protein-coding genes whose levels co-varied with, and potentially regulated, transposon levels."
In addition to studying the regulation aspect of transposons, the approach also enabled the study of potential age-related effects transposon dysregulation may have, he said.
"With the aid of our collaborators, we were able to determine whether transposon-linked genomic variation was previously linked to aging-associated diseases by other researchers," Bravo said. "And indeed, it had been."
Working with collaborators at Columbia University, the team cross-referenced the suspect genes with transcriptomic and genomic data from more than 400 individuals. The results paint a clear picture of the relationship between increased expression of LINE-1, expression of its suspected regulatory genes, and signs of accelerated aging.
"This study sets a new framework for studying transposons and adds to the body of evidence showing that transposable element activation is contributing to aging," Benayoun said.
She added that this study was also novel in part for focusing on transposon activity and regulation in somatic (body) cells, versus reproductive cells or cancer cells.
"The majority of everything we know about transposon regulation is from germline or cancer cells; there's nothing much that's been done to look at transposon regulation in normal somatic cells," she said.
Confirmation in the lab IDs potential aging targets
To support their findings, Benayoun and her team worked with human cells in vitro to see how overexpression of the suspected regulatory genes affected the activity of LINE-1. Engineering cells to overexpress two of the genes, IL16 and STARD5, markedly increased overall LINE-1 expression. In addition, treating normal cells with a short-term exposure to IL16 protein also induced higher expression of LINE-1.
"These are new validated regulators of transposable element activity, and they are potential targets for aging," Benayoun said.
Regulating jumping genes is a new job description for both of these genes, but their potential connections to aging make sense, Bravo said. STARD5 is involved in moving cholesterol within cells and is upregulated in response to stress in the endoplasmic reticulum (ER)—an organelle involved in protein synthesis and lipid metabolism.
"Aging is often accompanied by changes that can promote ER stress. Given its role, it's possible that STARD5 is involved in age-related alterations," Bravo said. "Interestingly, we observed that upregulating STARD5 led to an upregulation of IL16, suggesting that there may be a synergy between the two in activating transposons."
IL16 is mainly known for its role in regulating immune responses to infection, though they found that its blood levels increase with age. Connections between jumping gene activity and immune responses aren't far-fetched—evolutionary biology research has indicated that transposons are descendants of ancient viruses that took up residence in cells, Benayoun explained.
In addition, chronic low-grade inflammation is one of the signs of biological aging, which could be tied to immune responses to transposon expression.
In addition, previous studies have indicated a possible role in diseases of aging for another of the genes highlighted by this analysis, a variation of the gene HLA; however, current technical limitations prevented experimental validation alongside STARD5 and IL16, Benayoun explained. In any case, this study also serves as a proof of principle that this type of analysis is a powerful new technique in aging research, she added.
"We were able to find exciting new results using a 15-year-old data set," Benayoun said.
"This approach can be used on bigger cohorts of data, which opens the door to finding more of these regulators. From there, we might be able to identify actionable therapeutic targets for aging and age-related diseases."
More information: Juan I. Bravo et al, An eQTL-based approach reveals candidate regulators of LINE-1 RNA levels in lymphoblastoid cells, PLOS Genetics (2024). DOI: 10.1371/journal.pgen.1011311



















