(Medical Xpress)—Neurobiologists at the Friedrich Miescher Institute for Biomedical Research (FMI) are the first to show that directional migration of neurons during brain development is controlled through epigenetic processes. In an elaborate study bridging epigenetics and neurobiology, the scientists found that the migratory pattern is orchestrated through epigenetic regulation of genes within neurons and spatial signals in the environment. Their study has been published in Science.
As the foundation for our mind is laid, 100 billion cells are formed and appropriately connected in the brain. Despite the huge number of cells, no aspect of this process is left entirely to chance. Neurons divide, take on defined identities, migrate to the correct nodes in the network and send out their connecting axons along predefined paths to make contact with specific target neurons. The blueprint for these arrangements is encoded in the genome. However, how coordinated transcription of genes is finely tuned to achieve the precision of these processes is not yet clear.
A study by the research group of Filippo Rijli, group leader at the FMI and Professor of Neurobiology at the University of Basel, shows now for the first time that long-distance neuronal migration in the developing brain is regulated through transcriptional programs that are epigenetically controlled.
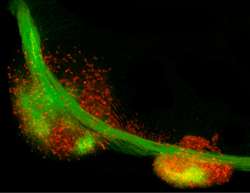
In their study published in Science, the neurobiologists have looked at a part of the brain called the brain stem and, in particular, at the neuronal ensembles forming the so-called precerebellar pontine nuclei. These nuclei are particularly important for the relay of information from the sensory and motor cortex to the cerebellum. During development, neurons, which will gather to form the pontine nuclei, migrate a long way from a distant progenitor compartment to their final positions, where they form connections that are vital for coordinated movement. The migratory path of these cells is defined by the relative position of the neuron in the progenitor compartment and is controlled by its specific combinatorial expression of Hox genes. Hox genes encode transcription factors and play an important role in many developmental processes that rely on a body plan and confer cellular identity.
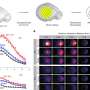
It has been known that neurons in the precerebellar pontine nuclei start to migrate in the wrong direction as soon as their Hox identity has been disrupted. The Rijli team has now shown that epigenetic processes control the maintenance of appropriate Hox expression during migration. The key player in this scenario is a major contributor to mammalian epigenetic control, the histone methyl-transferase Ezh2. Ezh2 methylates histones and silences specific stretches of DNA, thus maintaining certain Hox genes repressed, while allowing expression of others.
Ezh2 also regulates the appropriate response to environmental clues that direct neuronal migration. The cells in the brain stem bathe in a sea of attractants and repellants. They respond to these stimuli depending on their identity and adapt their migratory paths. Rijli and colleagues found that Ezh2 controls transcription of both environmental Netrin, a neuronal attractant molecule and of its repellant receptor Unc5b in migrating neurons, such that the appropriate balance between attraction and repulsion is maintained throughout migration to keep neurons on track.
"Being able to link epigenetic regulation with a complex process such as long-distance directional neuronal migration during brain development is extremely exciting," comments Rijli. "All the more we were delighted to see that the migratory pattern is not only epigenetically maintained through an intrinsic program established in the progenitor, but is also coordinated with an Ezh2-dependent silencing program that regulates the spatial distribution of extrinsic signals in the migratory environment. The knowledge gained from our studies contributes as well to our understanding of certain neurological syndromes that are caused by faulty neuronal migration and are currently incurable."
More information: Di Meglio T. et al., (2013) Ezh2 orchestrates topographic tangential migration and connectivity of mouse precerebellar neurons. Science, 339:204-207. www.sciencemag.org/content/339/6116/204.abstract
Journal information: Science