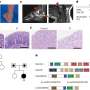
Scientists at The Royal Melbourne Hospital and the University of Melbourne in Australia have discovered the cells that cause a common type of childhood leukaemia - T cell Acute Lymphoblastic Leukaemia (T-ALL). Targeting of these cells may lead to improved treatments for this disease and help prevent relapse.
The team, led by Dr Matthew McCormack and Dr David Curtis of the Rotary Bone Marrow Research Laboratories and the University's Department of Medicine at The Royal Melbourne Hospital, made the discovery whilst studying mice prone to developing this leukaemia.
The results have been published online today by the prestigious international journal Science.
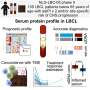
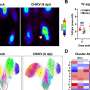
The team found that with irradiation treatment in animal models, over 99 per cent of cells in the thymus were killed, but these stem cell-like cells persisted and rapidly recovered. This suggests that these cells may survive therapy and be responsible for relapsed disease following treatment.
Currently, children with T-ALL are given extended therapy over two to three years in an attempt to stop a relapse. More targeted therapy on the thymus cells could reduce the length and toxicity of treatment and prevent relapse.
Dr McCormack, a leading international expert on childhood leukaemia, said: "The cellular origins of this leukaemia are not well understood. Our discovery that these cells are similar to normal stem cells explains why they are capable of surviving for long periods. It also explains why they are remarkably resistant to treatment."
Approximately 50 new cases of T-ALL are diagnosed every year in Australia, two thirds of these in children or adolescents. Adults also contract T-ALL, and the majority succumb to resistant or relapsed disease.
Dr Curtis, a Clinical Haematologist and head of the Leukaemia Research Program at The Royal Melbourne Hospital, said: "The identification of these cells provides an important target for the development and testing of new treatments for patients with T cell Acute Lymphoblastic Leukaemia."
The team will now focus on novel treatments capable of killing these cells, which may lead to clinical trials within the next five years.
Provided by University of Melbourne