Credit: AI-generated image (disclaimer)
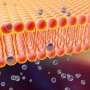
Helicobacter pylori, the leading cause of peptic ulcer disease and stomach cancer. One factor important to H. pylori infection is the pore-forming toxin VacA. It is thought to gain entry into host cells by binding to specialized membrane domains called lipid rafts.
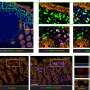
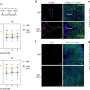
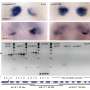
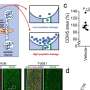
Using Giant Plasma Membrane Vesicles (GPMVs), Anne Kenworthy, Ph.D., and colleagues studied how VacA binds to cell membranes.
They found that wild-type VacA toxin predominantly associated with the raft phase of GPMVs. Mutant VacA proteins that did not form oligomers or membrane channels still associated with lipid rafts, and a partial domain of the VacA protein was also capable of binding to lipid rafts.
The study, reported in Infection and Immunity, provides a direct measure of VacA's association with lipid rafts and defines features of the protein that mediate its affinity for raft domains. The findings are important for understanding the toxin's function in H. pylori infection.
More information: Krishnan Raghunathan et al. Determinants of Raft Partitioning of the Helicobacter pylori Pore-Forming Toxin VacA, Infection and Immunity (2018). DOI: 10.1128/IAI.00872-17
Journal information: Infection and Immunity
Provided by Vanderbilt University