This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Limiting certain light exposure has potential to prevent inherited retinal dystrophy
A joint team effort led by Dr. Haruhisa Inoue (Professor, Department of Cell Growth and Differentiation, CiRA) has established iPS cells from two patients with EYS-associated retinal dystrophies (EYS-RD) and converted them into retinal organoids to study the root cause of this debilitating visual disability. The study is published in JCI Insight.
Inherited retinal dystrophies (IRDs) represent a collection of degenerative eye pathologies affecting photoreceptor cells in the retina that may ultimately result in irreversible vision loss. IRD is a leading cause of visual disability and blindness, affecting more than 4.5 million people worldwide, and is known to be caused by mutations in more than 250 genes.
Mutations in the eyes shut homolog (EYS) gene are a principal contributor to IRD cases. However, many prevailing animal models used for disease modeling are missing or have a disrupted version of the EYS gene in their genomes, thus making it challenging to model the disease in mammals.
Through zebrafish studies, EYS has been described to play a critical role in maintaining the morphological structure of photoreceptors and regulating protein transport to the outer segments of photoreceptor cells.
Nonetheless, it remains unclear whether such disrupted functions in IRD patients are responsible for disease and whether the disruption of such biological processes is responsible for IRD pathogenesis. Therefore, there is a need to create new experimental models using human cells to understand the disease and devise potential treatments.
To this end, the research team derived iPS cells from two unrelated EYS-RD patients with the same mutation in the EYS gene and generated retinal organoids to compare with ones made from healthy iPS cells to model the disease.
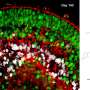
Initial morphological, histological (immunofluorescence and electron microscopy), and molecular (single-cell RNA sequencing) analysis of the EYS-RD retinal organoids suggest that the EYS mutation did not affect retinal development such that all cell types were present and all subcellular compartments present in photoreceptor cells formed properly.
However, even though the mutation did not affect the total amount of EYS protein, the research team noted less EYS protein, especially EYS-positive punctate structures, in the cilia or outer segment (OS) regions of photoreceptor cells in EYS-RD retinal organoids.
In contrast, the researchers examined the subcellular localization of PRPH2, a known OS marker, to find that it was not affected by the EYS mutation, thus suggesting PRPH2 is transported into the OS by an EYS-independent mechanism.
Because EYS and GRK7, a protein involved in the termination and recovery of the photoresponse, are both OS proteins predominantly expressed in cone cells (as compared to rod cells) and share a similar evolutionary history (both lost in some mammalian species), the two proteins might be functionally linked.
Remarkably, the researchers observed that GRK7 is significantly reduced, even absent in some cases, in the OS region of mutant retinal organoids. Conversely, they found that the EYS mutation of interest in this study did not affect the EYS-GRK7 protein–protein interaction, thus indicating the defect in GRK7 subcellular localization is primarily caused by EYS mislocalization rather than an inability to interact with GRK7 for transport to the OS region.
By expressing a normal version of EYS in EYS-RD retinal organoids, the research team successfully rescued these defects with GRK7 intracellular transport.
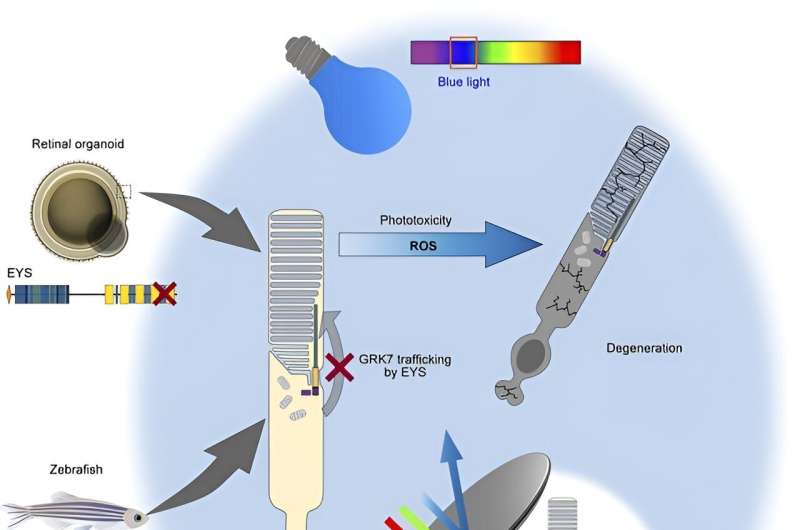
To validate these findings further, the research team deleted the EYS gene from healthy iPS cells and zebrafish to generate knockout (KO) human iPS cell-derived retinal organoids and zebrafish, respectively. Similar defects in GRK7 mislocalization were observed in both experimental systems, thus confirming the role of EYS in trafficking GRK7 and the loss of function caused by the EYS mutation identified in the patients.
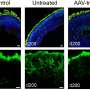
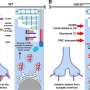
Given the role of GRK7 in terminating and mediating recovery of the photoresponse, the researchers next focused on light-induced changes in the zebrafish retina. Compared to eys-KO zebrafish kept in the dark, eys-KO mutants exposed to white LED light showed remarkably disrupted retinal structure, with a widespread loss of photoreceptors, predominantly affecting the cone cell populations.
An examination of cells undergoing a biological process called programmed cell death, or apoptosis, consistently showed a light-dependent increase in dying cells in eys-KO zebrafish. A biochemical assay further indicated light-induced overstimulation in the absence of eys, leading to increased production of reactive oxygen species that are detrimental to cells if not resolved by cytoprotective mechanisms.
The researchers also found light stimulation to trigger photoceptor cell death in EYS-KO retinal organoids and further showed that blue light is especially lethal.
Altogether, using iPS cell-derived retinal organoids and a zebrafish model system, the researchers have identified the pathogenic mechanism underlying retinal degeneration in EYS-RD. Although it remains to be seen how these findings can be translated practically as preventative care, the research team revealed the light-dependent cytotoxicity, especially by high-intensity blue light, in the absence of fully functional EYS protein, thus suggesting a potential way to safeguard against retinal degeneration is to limit light exposure by tinted glasses or other protective devices.
More information: Yuki Otsuka et al, Phototoxicity avoidance is a potential therapeutic approach for retinal dystrophy caused by EYS dysfunction, JCI Insight (2024). DOI: 10.1172/jci.insight.174179