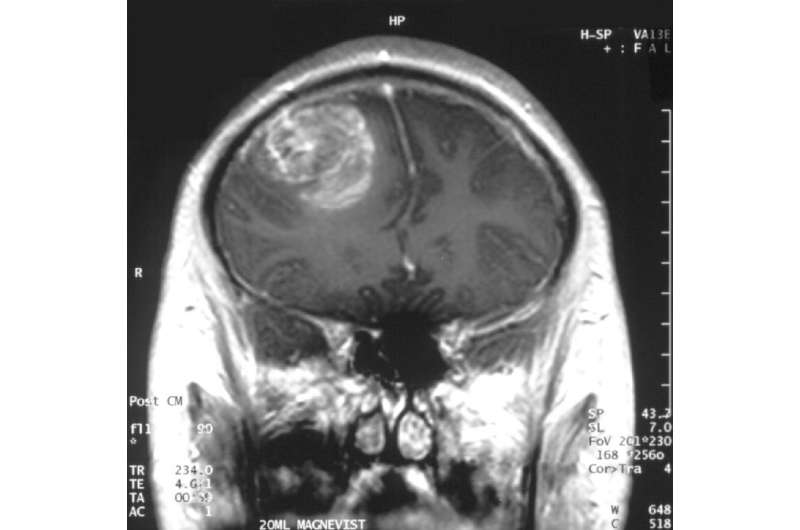
Gliobastoma (astrocytoma) WHO grade IV—MRI coronal view, post contrast. 15 year old boy. Credit: Christaras A/ Wikipedia.
Inhibiting a novel protein variant within glioma stem cells may be a promising therapeutic approach to treat glioblastoma, according to a Northwestern Medicine study published in Nature Cell Biology.
"With this study, we found a novel peptide, which is only highly expressed in cancer, activates the EGFR signaling pathway," said Shi-Yuan Cheng, Ph.D., professor in the Ken and Ruth Davee Department of Neurology Division of Neuro-Oncology and co-senior author of the study.
Glioblastoma, the most common and aggressive type of brain cancer, is associated with an average survival rate of 12 to 18 months, due to the tumor's high plasticity which limits the effectiveness of current therapies.
Roughly half of glioblastoma tumors are associated with increased activation of the EGFR cell signaling pathway in tumor cells, a known oncogenic driver of tumor growth. However, previous work has found EGFR-targeting therapies are ineffective in treating patients with glioblastoma, emphasizing the need for novel therapeutic interventions.
For the current study, the investigators aimed to identify coding circular RNAs (circRNAs)—strands of RNA that form closed loops and are involved in various physiological processes—in glioblastoma tumor cells, and whether multiple rounds of circRNA translation generates proteins that could serve as potential therapeutic targets.
Using RNA sequencing and ribosomal profiling of paired normal and tumor tissue samples from vitro and in vivo models of glioblastoma, the team found that a novel E-cadherin protein variant called C-E-Cad is overexpressed in glioma stem cells.
Specifically, C-E-Cad binds to EGFR through a novel and unique 14 amino acid sequence at its tail and activates the EGFR signaling pathway independent of EGF, the prototype ligand that activates EGFR, thereby promoting cell proliferation and overall glioblastoma tumor growth.
Additionally, the team found that inhibiting C-E-Cad enhanced EGFR-targeting therapies, suggesting the approach may be promising for treating EGFR-driven glioblastoma.
"A specific anti-E-C-Cad antibody against this new 14 amino acid sequence, together with EGFR antibodies, enhances tumor suppression," said Cheng, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
More information: Xinya Gao et al. Circular RNA-encoded oncogenic E-cadherin variant promotes glioblastoma tumorigenicity through activation of EGFR–STAT3 signalling, Nature Cell Biology (2021). DOI: 10.1038/s41556-021-00639-4
Provided by Northwestern University