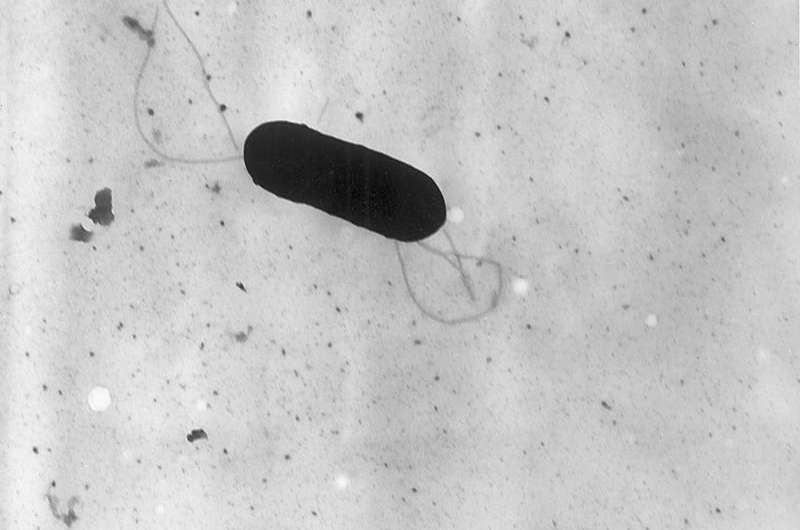
Electron micrograph of a flagellated Listeria monocytogenes bacterium, Magnified 41,250X. Credit: CDC/public domain
The protein kinase CK2 is involved in a wide range of biological processes and cellular functions, including inflammatory responses and pathologies associated with inflammation. Also, its aberrant expression and activity are characteristic of many cancers. Yet the basic function of CK2 in CD8+ immune T cells has remained completely unknown.
CD8+ T cells, also known as killer T cells, are critical to maintain health by controlling infections by intracellular pathogens, including viruses and intracellular bacteria. CD8+ T cells also can control cancer progression by directly killing cancer cells, and through recruiting and activating other immune cells.
Now, in a study published in the Journal of Immunology, University of Alabama at Birmingham researchers report that protein kinase CK2 controls CD8+ T cell effector and memory functions during infection. These experiments were done in mouse CD8+ T cells and a mouse model of infection by the intracellular pathogenic bacteria Listeria monocytogenes.
The CK2 kinase is known to act in cellular signaling pathways by phosphorylating target proteins at specific serine or threonine amino acids. The CK2 kinase has two catalytic subunits and two regulatory subunits. The UAB researchers, led by Hongwei Qin, Ph.D., and Etty "Tika" Benveniste, Ph.D., deleted the CK2α catalytic subunit from mouse CD8+ T cells and compared them to CD8+ T cells with an intact CK2 kinase.
The researchers first showed that the CK2 subunits were induced, and that CK2 kinase activity was increased, when intact CD8+ T cells were activated by stimulating the T cell receptor to trigger an immune response. That showed a potential role of CK2 in CD8+ T cell responses.
In contrast, the CK2α-/- deletion mutants showed significantly less CD8+ T cell activation and proliferation, indicating that the CK2α activity was required for those steps.
Furthermore, the researchers say their findings suggest that CK2α is involved in CD8+ T cell metabolic reprogramming during activation, specifically through increases in glycolytic metabolism and mitochondrial respiration. Mitochondria are the powerhouses of cells, supplying most of the cellular energy. Upon activation, intact T cells are known to undergo substantial metabolic reprogramming; but the UAB researchers found that CK2α-/- deletion mutants had significantly less glucose uptake and metabolism, and decreased mitochondrial respiration, compared to T cells with normal CK2.
Mechanistically, the UAB team showed that CK2α controlled the reprogramming of CD8+ T cell metabolism by regulating the well-known AKT/mTOR signaling pathway.
Using a mouse Listeria monocytogenes infection model, Qin, Benveniste and colleagues found that CK2α was required for CD8+ T cell expansion, maintenance and effector function in both primary and memory stages during infection by the intracellular bacterial pathogen.
"Taken together, our study demonstrates that CK2α regulates CD8+ T cell activation and differentiation both in vitro and in vivo," Benveniste said. "However, the biological function of CK2α in CD8+ T cells, particularly CD8+ T cell cytotoxic function, remains to be explored."
More information: Wei Yang et al, Protein Kinase CK2 Controls CD8+T Cell Effector and Memory Function during Infection, The Journal of Immunology (2022). DOI: 10.4049/jimmunol.2101080
Journal information: Journal of Immunology
Provided by University of Alabama at Birmingham