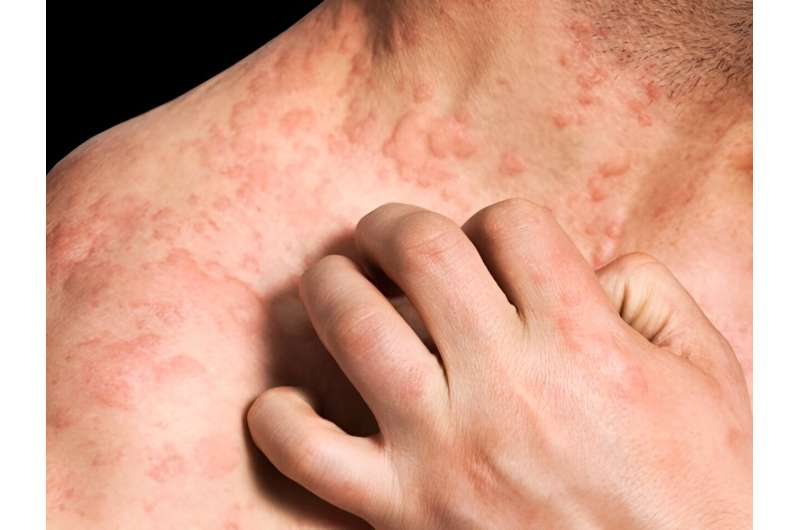
For patients with moderate-to-severe prurigo nodularis, nemolizumab improves symptoms, according to a study published in the Oct. 26 issue of the New England Journal of Medicine.
Shawn G. Kwatra, M.D., from the Johns Hopkins University School of Medicine in Baltimore, and colleagues conducted a phase 3, double-blind, randomized trial involving adults with moderate-to-severe prurigo nodularis. Participants were assigned to receive an initial 60-mg dose of nemolizumab followed by subcutaneous injections of 30 or 60 mg every four weeks for 16 weeks or matching placebo (183 and 91 patients, respectively).
The researchers found that at week 16, treatment efficacy was shown with respect to both primary end points (itch response and Investigator's Global Assessment [IGA] response); a greater proportion of patients in the nemolizumab group than in the placebo group had an itch response (56.3 versus 20.9%) and an IGA response (score of 0 [clear] or 1 [almost clear]; 37.7 versus 11.0%). For the five key secondary end points, benefits were also observed: itch response at week 4, Peak Pruritus Numerical Rating Scale score of less than 2 at weeks four and 16, and improvement of 4 or more points on the sleep disturbance numerical rating scale at weeks four and 16.
"The results of this trial extend the efficacy and safety findings of nemolizumab from the phase 2 study in patients with prurigo nodularis," the authors write.
More information: Shawn G. Kwatra et al, Phase 3 Trial of Nemolizumab in Patients with Prurigo Nodularis, New England Journal of Medicine (2023). DOI: 10.1056/NEJMoa2301333
Martin Metz, Targeting Interleukin-31 in Prurigo, New England Journal of Medicine (2023). DOI: 10.1056/NEJMe2307584
Journal information: New England Journal of Medicine
Copyright © 2023 HealthDay. All rights reserved.