This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Examining the mechanisms and clinical potential of a promising non-opioid pain therapy candidate
A recent publication in Scientific Reports unveils a promising non-opioid pain treatment developed by a team led by Dr. Hernan Bazan, the John Ochsner Endowed Professor of Cardiovascular Innovation at Ochsner Health.
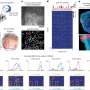
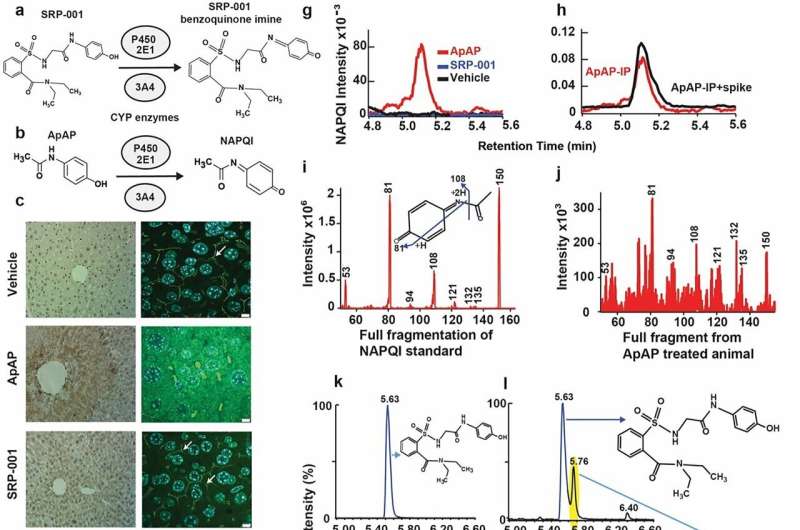
The study, titled "Transcriptomic Signature, Bioactivity, and Safety of an Analgesic Generating AM404 in the Midbrain PAG Region," introduces SRP-001, a novel medication in development by South Rampart Pharma, Inc., co-founded by Dr. Bazan.
The research publication delineates the unique biological mechanisms and clinical potential of SRP-001, highlighting its significance in addressing the critical health care challenge of safe and effective pain management.
Dr. Bazan said, "This groundbreaking research opens new pathways for understanding pain management and offers a promising avenue for developing more effective treatments. Publication in Scientific Reports underlines the innovative strides our team is making in advancing non-opioid pain management, potentially transforming the field."
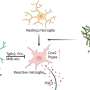
SRP-001 leverages unique biological mechanisms to alleviate pain by activating specific brain networks and enhancing AM404 production in the midbrain's periaqueductal gray (PAG) area. This mechanism significantly improves pain modulation without the typical side effects associated with traditional pain medications.
Results from the first-in-human Phase 1 trial confirm SRP-001's safety and tolerability, with promising pharmacokinetic profiles that support its progression to further clinical stages. The FDA granted Fast Track designation to SRP-001 for treating acute pain as of October 2023. SRP-001 is being considered for various pain conditions, including neuropathic, acute, chronic pain, and migraine headaches.
SRP-001 emerges as a pioneering non-opioid, non-hepatotoxic pain therapy candidate, providing a viable alternative to ApAP, NSAIDs, and opioids. Its unique mechanism, robust safety profile, and effective pain modulation capabilities position SRP-001 as a promising solution for safer pain treatment. The research underscores the need for continued exploration of SRP-001's therapeutic potential, paving the way for advancements in pain management strategies.
More information: Hernan A. Bazan et al, Transcriptomic signature, bioactivity and safety of a non-hepatotoxic analgesic generating AM404 in the midbrain PAG region, Scientific Reports (2024). DOI: 10.1038/s41598-024-61791-z