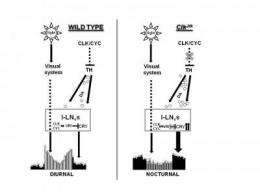
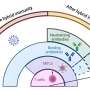
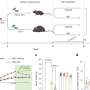
In wild type flies (left), light promotes activity during the day. These effects of light are likely mediated by the visual system (shown by dotted lines). In addition, the Clock protein suppresses dopamine signaling, which would otherwise promote activity at night. Clock also suppresses CRY. In Clock mutant flies (right), dopamine signaling is elevated (increased extracellular dopamine, circles), which acts through CRY to drive activity at night. Credit: Amita Sehgal, Ph.D., Perelman School of Medicine, University of Pennsylvania; Genes and Development
Perelman School of Medicine at the University of Pennsylvania researchers have discovered a mechanism involving the neurotransmitter dopamine that switches fruit fly behavior from being active during the day (diurnal) to nocturnal. This change parallels a human disorder in which increased agitation occurs in the evening hours near sunset and may also be due to higher than normal dopamine levels in the brain. Sundown syndrome occurs in older people with dementia or cognitive impairment.
Many geriatricians have noted an association between sundown syndrome and changes in the internal biological clock among people with dementia, observing disruptions in their sleep-wake cycles. The internal clock, which guides rhythms over a 24-hour day, is connected to how active humans are at different times of the day.
The lab of Amita Sehgal, PhD, professor of Neuroscience and a Howard Hughes Medical Institute investigator, found that in fruit flies dopamine acts to arouse flies through a protein called cryptochrome that normally functions as a photoreceptor. Flies, like humans, are typically diurnal. The findings were released online this week in Genes & Development.
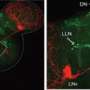
Using a strain of fly that has a mutated Clock (Clk) gene and exhibits nocturnal behavior, the team found that increased night-time activity of the Clk mutant flies is associated with elevated dopamine signaling. Specifically, Clk mutant flies have increased levels of an enzyme used to make dopamine called tyrosine hydroxylase in fly brains. The night-time behavior also requires the circadian photoreceptor, cryptochrome (CRY), in specific cells of the fly brain. These cells also express CLK, as do others that may be responsible for the elevated dopamine.
"Our results suggest that typically the dopamine pathway and CRY promote acute arousal. They allow an animal to respond to acute stimulation by sensory stimuli with a transient increase in activity," says Sehgal. "However, chronic, increased signaling of the pathway leads to nocturnal activity, most likely because both CRY and dopamine activity are dampened by light." A switched behavioral cycle is the result.
Based upon previous research and the present study, the Sehgal team proposed that CRY is required for two distinct acute responses -- acute arousal, which presumably awakens the animal, and resetting of the circadian clock in response to a pulse of light. This would fit with evolutionary needs as, even when they're sleeping, animals need to be able to respond to sudden changes in the environment. It appears that both arousal, as well as circadian responses, to such sudden stimulation require the same molecule, says Sehgal.
The researchers also note parallels to these findings in mammals. Dopamine in mammals regulates melanopsin, a pigment in the retina important to synchronizing circadian cycles, much like CRY does in flies. Light-sensitive melanopsin is required to induce sleep in nocturnal rodents, who sleep during the day. In contrast, in humans, who are diurnal, mistimed elevated dopamine is linked to increased agitation and sleep disturbances in the early evening – the so-called sundown syndrome. "Although there are differences in CRY signaling between flies and humans, it is interesting to note that sundown syndrome is treated with medications that decrease the activity of dopamine," says Sehgal.
She notes that the next step in this work would be to determine the molecular connection between dopamine and CRY and also to identify the mechanism by which CRY promotes arousal.
Provided by University of Pennsylvania School of Medicine