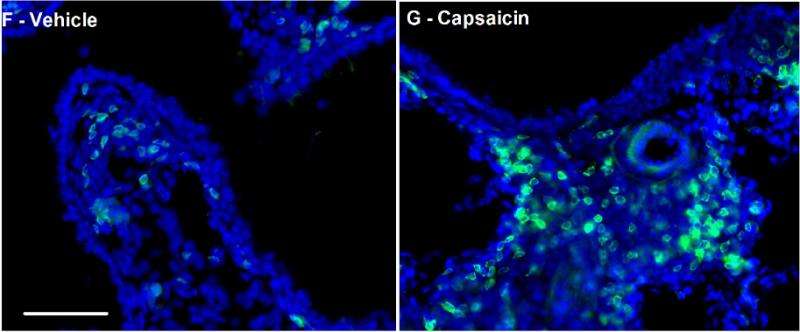
Fig 1 F/G: Activation of nociceptors, done for study purposes with a compound called capsaicin, promotes the infiltration of immune cells into the lung (right) as compared with control (left). Credit: Boston Children's Hospital
Current asthma medications, which work by suppressing inflammatory signaling by immune cells or by dilating the airways, can stop working over time. A study from Boston Children's Hospital, Brigham and Women's Hospital, and Harvard Medical School supports a surprising alternative approach to controlling asthma: targeting certain sensory nerve endings in the lungs that help drive allergic inflammation.
Reporting online in the journal Neuron on June 25, the researchers show that specialized sensory neurons called nociceptors are not only activated by allergic inflammation, but also exacerbate the allergic immune response. When these neurons are selectively silenced in mouse models of acute and chronic asthma, both inflammation and bronchial twitchiness are reduced.
Nociceptors in our lungs connect to the brainstem and trigger the cough reflex when they detect potential harms like dust particles, chemical irritants or allergens. Nociceptor nerve endings are known to be more plentiful and more readily activated in people with asthma, but a role in driving allergic inflammation had not previously been suspected.
"An attractive aspect of targeting nociceptors is that this approach would be most effective when inflammation is already present and should accelerate its resolution," says Clifford Woolf, M.D., Ph.D., director of the F.M. Kirby Neurobiology Center at Boston Children's Hospital and a co-senior investigator on the study.
"Current asthma treatments can help to control symptoms and dampen airway inflammation; however, therapies are not available to promote the resolution of asthma," says co-senior investigator Bruce Levy, M.D., chief of the Pulmonary and Critical Care Medicine Division at Brigham and Women's Hospital. "A treatment to interrupt the vicious cycle of neuro-immune signaling holds promise as a disease-modifying therapy and is mechanistically distinct from any of the currently available asthma therapies."
The research team, led by first author Sébastien Talbot, Ph.D., of Boston Children's, tested a strategy for selectively blocking nociceptor activity in mice that was developed by the Woolf lab with Bruce Bean, Ph.D. at Harvard Medical School, using a drug called QX-314.
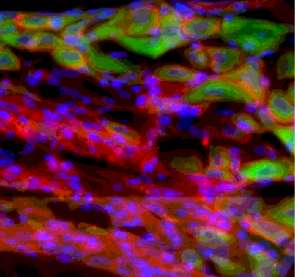
Fig 7B: Lung inflammation and release of IL-5 activates nociceptors to produce VIP (shown in green). Credit: Boston Children's Hospital
"There was prior evidence that neurons and the immune system talk to each other," explains Talbot. "Since we have QX-314, which specifically blocks pain neurons that are activated by inflammation, we wanted to see if such interplay also occurs in asthma."
In the study, they induced asthma in mice by exposing them to dust mites or another allergen, ovalbumin, then administered QX-314 via nebulizer to silence the nociceptors. These were their findings:
- When stimulated, nociceptors release chemicals (neuropeptides) that cause immune cells to infiltrate the lungs and become more active.
- IL-5, an inflammatory molecule produced by the immune cells, in turn activates the nociceptors to produce a neuropeptide called vasoactive intestinal peptide (VIP).
- VIP further stimulates the inflammatory response, creating a neuro-immune feedback loop that inflames the lungs and escalates asthma symptoms.
- When asthmatic mice had their nociceptors silenced, either genetically or with QX-314, they had much less airway inflammation and less bronchial twitchiness.
The researchers conclude that nociceptors both react to and drive inflammatory immune responses in the lung, and that silencing these cells interrupts this feedback loop, helping relieve allergic airway inflammation and bronchospasm.
QX-314 is chemically related to the local anesthetic lidocaine, modified in such a way that it specifically targets inflammation-activated nociceptors (including those in the lung) and stays inside cells for prolonged periods without getting into the bloodstream. The researchers believe these properties will increase the drug's duration of action in the lung and limit side effects.
"QX-314 is not able to get into nerve cells normally, but it can enter cells by passing through the large pores of TRP-family ion channels, which are expressed selectively in nociceptors and are activated during inflammation," explains Bean, also a professor of neurobiology at Harvard Medical School. "This limits the action of QX-314 to just the neurons activated by inflammation."
"People have tried to use lidocaine itself to target asthma, but it works nonspecifically on all neurons, posing a risk of impaired swallowing," notes Talbot. "It can also spread to and act on the heart and brain."
With the help of the Harvard and Boston Children's technology development offices, the Woolf and Bean labs are working on new, more potent versions of QX-314 that would enhance its safety but preserve and even increase its beneficial properties.
"We are sufficiently encouraged by the strong relief of lung inflammation and airway constriction to actively embark on a major drug development program, with the aim of clinically testing this strategy for multiple allergic conditions," says Woolf.
Journal information: Neuron
Provided by Children's Hospital Boston